Abstract
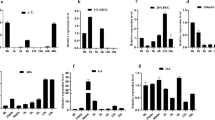
Thermosensitive male sterility plays an important role in wheat fertility and production. As a key enzyme for chlorophyll degradation, pheophorbide a oxygenase (PaO) can suppress cell death in plants. We cloned the wheat gene TaPaO1 from the thermosensitive genetic male sterile (TGMS) line BS366; it encodes a typical PaO protein, containing a conserved Rieske [2Fe-2S] iron–sulphur motif, a mononuclear non-heme iron-binding motif, and a C-terminal CxxC motif. TaPaO1 was expressed in all tissues and was upregulated during the meiosis stage of BS366 anthers under low temperature. Subcellular localization of TaPaO1 specifically labelled the surrounding of chloroplasts. TaPaO1 regulated by RD29A promoter which responded to low temperature led to pollen sterility in transgenic tobacco. Expression analysis showed that TaPaO1 exhibited a higher level of expression in the anther than in other tissues in transgenic tobacco plants during low temperature treatment. We propose that the higher senescence-related activity of TaPaO1 may lead to the cell death of anthers, which happens at an early developmental stage under low temperature. These results provide new insights into the function of PaO during the early developmental stage of anthers. PaO is closely related to cell death regardless of whether it exhibits increased activity or inactive.
Similar content being viewed by others
References
Buchanan-Wollaston V (1997) The molecular of leaf senescence. J Exp Bot 48:181–199
Chen L, Liu YG (2014) Male Sterility and Fertility Restoration in Crops. Annu. Rev. Plant Bio l65:579–606
Gray J, Buckner DJ, Buckner B, Close PS, Johal GS (2002) Lightdependent death of maize lls1 cells is mediated by mature chloroplasts. Plant Physiol 130:1894–1907
Gray J, Close PS, Briggs SP, Johal GS (1997) A novel suppressor of cell death in plants encoded by the Lls1 gene of maize. Cell 89: 25–31
Greenberg JT, Ausubel FM (1993) Arabidopsis mutants compromised for the control of cellular damage during pathogenesis and aging. Plant J 4:327–341
Guttman M, Rinn JL (2012) Modular regulatory principles of large non-coding RNAs. Nature 482:339–346
Hörtensteiner S, Kra¨utler B (2011) Chlorophyll breakdown in higher plants. Biochim. Biophys. Acta 1807:977–988
Hörtensteiner S (1999) Chlorophyll breakdown in higher plants and algae. Cell Mol Life Sci 56:330–347
Hörtensteiner S, Rodoni S, Schellenberg M, Vicentini F, Nandi OI, Qiu YL, Matil P (2000) Evolution of chlorophyll degradation: the significance of RCC reductase. Plant Biol 2:63–67
Hörtensteiner S, Wüthrich KL, Matile P, Ongania KH, Kräutler B (1998) The key step in chlorophyll breakdown in higher plants. Cleavage of pheophorbide a macrocycle by a monooxygenase. J Biol Chem 273:15335–15339
Joyard J, Ferro M, Masselon C, Seigneurin-Berny D, Salvi D, Garin J, and Rolland N. (2009) Chloroplast proteomics and the compartmentation of plastidial isoprenoid biosynthetic pathways. Mol. Plant 2:1154–1180
Li P, Ma Y, Li X, Zhang L, Wang Y, Wang N (2006) Cloning and expressional characterization of soybean GmLls1 gene. Chin Sci Bull 51:1210–1218
Mach JM, Castillo AR, Hoogstraten R, Greenberg JT (2001) The Arabidopsis-accelerated cell death gene ACD2 encodes red chlorophyll catabolite reductase and suppresses the spread of disease symptoms. Proc. Natl. Acad. Sci. USA. 98:771–776
Ma N, Ma X, Li AF, Cao XC, Kong LR (2012) Cloning and expression analysis of wheat pheophorbide a oxygenase gene TaPaO. Plant MolBiol Rep 30:1237–1245
Matile, P and Schellenberg, M (1996) The cleavage of pheophorbide a is located in the envelope of barley gerontoplasts. Plant Physiol.Biochem 34:55–59
Murray M, Thompson WF (1980) Rapid isolation of high molecular weight plant DNA. Nucleic Acids Res 8:4321–4326
Nakashima K, Fujita Y, Katsura K, Maruyama K, Narusaka Y, Seki M, Yamaguchi-Shinozaki K (2006) Transcriptional regulation of ABI3-and ABA-responsive genes including RD29B and RD29A in seeds, germinating embryos, and seedlings of Arabidopsis. Plant Mol Biol 60:51–68
Peterson R, Slovin JP, Chen CB (2010) A simplifed method for differential staining of aborted and non-aborted pollen grains. Internation of Plant Biology 1: e13
Pruźinská A, Tanner G, Anders I, Roca M, Hörtensteiner S (2003) Chlorophyll breakdown: Pheophorbide a oxygenase is a Riesketype iron-sulfur protein, encoded by the accelerated cell death 1 gene. Proc. Natl. Acad. Sci. USA. 100: 15259–15264
Pruźinská A, Tanner G, Aubry S, Anders I, Moser S, Müller T, Ongania KH, Kraütler B, Youn JY, Liljegren SJ, and Hörtensteiner S (2005) Chlorophyll breakdown in senescent Arabidopsis leaves. Characterization of chlorophyll catabolites and of chlorophyll catabolic enzymes involved in the degreening reaction. Plant Physiol 139:52–63
Sakuraba Y, Schelbert S, Park SY, Han SH, Lee BD, Andrès CB, Kessler F, Hörtensteiner S and Paek NC (2012) Stay-green and chlorophyll catabolic enzymes interact at light-harvesting complex II for chlorophyll detoxification during leaf senescence in Arabidopsis. Plant Cell 24:507–518
Spassieva S, Hille J (2002) A lesion mimic phenotype in tomato obtained by isolating and silencing an Lls1 homologue. Plant Sci 162:543–549
Tanaka R, Hirashima M, Satoh S, Tanaka A (2003) The Arabidopsis-accelerated cell death gene ACD1 is involved in oxygenation of pheophorbide a: inhibition of pheophorbide a oxygenase activity does not lead to the “stay-green” phenotype in Arabidopsis. Plant Cell Physiol 44:1266–1274
Tang Y, Li M, Chen Y, Wu P, Wu G, Jiang H (2011) Knockdown of OsPAO and OsRCCR1 cause different plant death phenotypes in rice. J Plant Physiol 168:1952–1959
Tang Z, Zhang L, Yang D, Zhao C, Zheng Y (2011) Cold stress contributes to aberrant cytokinesis during male meiosis I in a wheat thermosensitive genic male sterile line. Plant Cell Environ 34:389–405
Yamaguchi-Shinozaki K, Koisumi M, Urao S, Shinozaki K (1992) Molecular cloning and characterization of 9 cDNAs genes that are responsive to desiccation in Arabidopsis thaliana: sequence analysis of one cDNA clone that encodes a putative transmembrane channel protein. Plant Cell Physiol 33:217–224
Yamaguchi-Shinozaki K, Shinozaki K (1993a) Arabidopsis DNA encoding two desiccation-responsive rd29 genes. Plant Physiol 101:1119–1120
Yamaguchi-Shinozaki K, Shinozaki K (1993b) Characterization of the expression of a desiccation-responsive rd29 gene of Arabidopsis thaliana and analysis of its promoter in transgenic plants. Mol Gen Genet 236:331–340
Yang M, Wardzala E, Johal GS, Gray J (2004) The wound-inducible Lls1 gene from maize is an orthologue of the Arabidopsis Acd1 gene, and the LLS1 protein is present in non-photosynthstic tissues. Plant Mol Biol 54:175–191
Zhang X, Li J, Liu A, Zou J, Zhou X, Xiang J, Rerksiri W, Peng Y, Xiong X, Chen X (2012) Expression profile in rice panicle: insights into heat response mechanism at reproductive stage. PLoS One 7: e49652
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Yuan, G., Wang, Y., Yuan, S. et al. Functional Analysis of Wheat TaPaO1 Gene Conferring Pollen Sterility Under Low Temperature. J. Plant Biol. 61, 25–32 (2018). https://doi.org/10.1007/s12374-017-0269-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12374-017-0269-7