Abstract
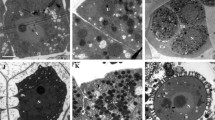
In the recessive genic male sterile line 9012A of Brassica napus, pollen development is affected during the tetrad stage. According to the light and electron microscopy analysis of tapetal cells and tetrads, the sterile tapetal cells swelled with expanded vacuoles at the early tetrad stage and finally filled the center of the locules where a majority of tetrads encased with the thick callose wall collapsed and degraded. We suggested that an absence of callase, which is a wall-degrading enzyme stored in the vacuoles of tapetal cells before secretion, resulted in the failure of tetrad separation. Moreover, transmission electron microscopy analysis showed that the secretory tapetal cells were not observed in sterile anthers, which indicated that the transition of the tapetum from the parietal type to the secretory type was probably aberrant. In plants, degeneration of the tapetum is thought to be the result of programmed cell death (PCD). PCD of tapetal cells was investigated by terminal deoxynucleotidyl transferase-mediated dUTP nick-end labeling assay and signals indicative of deoxyribonucleic acid fragmentation were detected much earlier in sterile anther than in fertile anther. This suggests that tapetal breakdown does not occur by the normal procession of PCD and might be following an alternative mechanism of unscheduled apoptosis in line 9012A. This research supports the hypothesis that premature PCD is associated with male sterility in B. napus.








Similar content being viewed by others
References
Balk J, Leaver CJ (2001) The PET1-CMS mitochondrial mutation in sunflower is associated with premature programmed cell death and cytochrome c release. Plant Cell 13:1803–1818
Budar F, Pelletier G (2001) Male sterility in plants: occurrence, determinism, significance and use. Life Sci 324:543–550
Carter C, Pan S, Zouhar J, Avila EL, Girke T, Raikhel NV (2004) The vegetative vacuole proteome of Arabidopsis thaliana reveals predicted and unexpected proteins. Plant Cell 16:3285–3303
Chen F, Hu BC, Li QS (1993) Discovery & study of genic male sterility (GMS) material “9012A” in Brassica napus L. Acta Scientarium Naturalium Universitatis Pekinensis 19(suppl):57–61
Chen FX, Hu BC, Li C, Li QS, Chen WS, Zhang ML (1998) Genetic studies on GMS in Brassica napus L. Inheritance of recessive GMS line 9012A. Acta Agronomica Sinica 24:431–438
De DN (2000) Plant cell vacuoles. CSIRO, Collingwood
Dong XY, Hong ZL, Sivaramakrishnan M, Mahfouz M, Verma DPS (2005) Callose synthase (CalS5) is required for exine formation during microgametogenesis and for pollen viability in Arabidopsis. Plant J 42:315–328
Fei H, Sawhney VK (1999) Ms32-regulated timing of callose degradation during microsporogenesis in Arabidopsis is associated with the accumulation of stacked rough ER in tapetal cells. Sex Plant Reprod 12:188–193
Goldberg RB, Beals TP, Sanders PM (1993) Anther development: basic principles and practical applications. Plant Cell 5:1217–1229
He JP, Ke LP, Hong DF, Xie YZ, Wang GC, Liu PW, Yang GS (2008) Fine mapping of a recessive genic male sterility gene (Bnms3) in rapeseed (Brassica napus) with AFLP- and Arabidopsis-derived PCR markers. Theor Appl Genet 117:11–18
Hong Z, Delauney AJ, Verma DPS (2001) A cell-plate specific callose synthase and its interaction with phragmoplastin. Plant Cell 13:755–768
Horner HT, Palmer RG (1995) Mechanisms of genic male sterility. Crop Sci 35:1527–1535
Hou GZ, Wang H, Zhang RM (1990) Genetic study on genic male sterility (GMS) material No. 117A in Brassica napus L. Oil Crops of China 2:7–10
Ito T, Shinozaki K (2002) The MALE STERILITY1 gene of Arabidopsis, encoding a nuclear protein with a PHD-finger motif, is expressed in tapetal cells and is required for pollen maturation. Plant Cell Physiol 43:1285–1292
Izhar S, Frankel R (1971) Mechanism of the male sterility in Petunia: the relationship between pH, callase activity in anthers, and the breakdown of the microsporogenesis. Theor Appl Genet 41:104–108
Johns CW, Delannay X, Palmer RG (1981) Structural sterility controlled by nuclear mutations in angiosperms. Nucleus (Calcutta) 24:97–105
Jung KH, Han MJ, Lee YS, Kim YW, Hwang I, Kim MJ, Kim YK, Nahm BH, An G (2005) Rice UNDEVELOPED TAPETUM1 is a major regulator of early tapetum development. Plant Cell 17:2705–2722
Kato T, Morita MT, Fukaki H, Yamauchi Y, Uehara M, Niihama M, Tasaka M (2002a) SGR2, a phospholipase-like protein, and ZIG/SGR4, a SNARE, are involved in the shoot gravitropism of Arabidopsis. Plant Cell 14:33–46
Kato T, Morita MT, Tasaka M (2002b) Role of endodermal cell vacuoles in shoot gravitropism. J Plant Growth Regul 21:113–119
Kaul MLH (1988) Male sterility in higher plants. Monographs on theoretical and applied genetics 10. Springer, New York, p 10
Kawanabe T, Ariizumi T, Kawai-Yamada M, Uchimiya H, Toriyama K (2006) Abolition of the tapetum suicide program ruins microsporogenesis. Plant Cell Physiol 47:784–787
Ke LP, Sun YQ, Liu PW, Yang GS (2004) Identification of AFLP fragments linked to one recessive genic male sterility (RGMS) in rapeseed (Brassica napus L.) and conversion to SCAR markers for marker-aided selection. Euphytica 138:1–6
Knapp SJ, Cox TS (1988) S1 family recurrent selection in autogamous crops based on dominant genetic male-sterility. Crop Sci 28:227–231
Li SL, Zhou XR, Zhou ZJ, Qian YX (1990) Inheritance of genetic male sterility (GMS) and its utilization in rape (Brassica napus L.). Crop Res 4:27–32
Li SL, Zhou ZJ, Zhou XR (1993) Inheritance of recessive genic male sterile line S45AB of rape (Brassica napus L.). Acta Agriculturae Shanghai 9:1–7
Li SL, Zhou ZJ, Zhou XR (1995) Three-line method of genetic male sterility for hybrid seed production in Brassica napus L. Acta Agriculturae Shanghai 11:21–26
Li N, Zhang DS, Liu HS (2006) The rice tapetum degeneration retardation gene is required for tapetum degradation and anther development. Plant Cell 18:2999–3014
Millar AA, Gubler F (2005) The Arabidopsis GAMYB-like genes, MYB33 and MYB65, are microRNA-regulated genes that redundantly facilitate anther development. Plant Cell 17:705–721
Morita MT, Kato T, Nagafusa K, Saito C, Ueda T, Nakano A, Tasaka M (2002) Involvement of the vacuoles of the endodermis in the early process of shoot gravitropism in Arabidopsis. Plant Cell 14:47–56
Owen HA, Makaroff CA (1995) Ultrastructure of microsporogenesis and microgametogenesis in Arabidopsis thaliana (L.) Heynh. ecotype Wassilewskija (Brassicaceae). Protoplasma 185:7–21
Pacini E, Franchi GG, Hesse M (1985) The tapetum: its form, function and possible phylogeny in Embryophyta. Plant Syst Evol 149:155–185
Rhee SY, Somerville CR (1998) Tetrad pollen formation in Quartet mutants of Arabidopsis thaliana is associated with persistence of pectic polysaccharides of the pollen mother cell wall. Plant J 15:79–88
Sanders PM, Anhthu QB, Weterings K, McIntire KN, Hsu Y, Lee PY, Troung MT, Beals TP, Goldberg RB (1999) Anther development defects in Arabidopsis thaliana male-sterile mutants. Sex Plant Reprod 11:297–322
Sanders PM, Lee PY, Biegsen C, Boone JD, Beals TP, Weile EW, Goldberg RB (2000) The Arabidopsis delayed dehiscence1 gene encodes an enzyme in the jasmonic acid synthesis pathway. Plant Cell 12:1041–1062
Scott RJ, Spielman M, Dickinson HG (2004) Stamen structure and function. Plant Cell 16(Suppl):S46–S60
Sorensen A, Guerineau F, Canales-Holzeis C, Dickinson HG, Scott RJ (2002) A novel extinction screen in Arabidopsis thaliana identifies mutant plants defective in early microsporangial development. Plant J 29:581–594
Sorrells ME, Fritz SE (1982) Application of a dominant male-sterile allele to the improvement of self-pollinated crops. Crop Sci 22:1033–1035
Steiglitz H (1977) Role of b-1,3-glucanase in postmeiotic microspore release. Dev Biol 57:87–97
Steiglitz H, Stern H (1973) Regulation of b-1,3-glucanase activity in developing anthers of Lilium. Dev Biol 34:169–173
Surpin M, Zheng H, Morita MT, Saito C, Avila E, Blakeslee JJ, Bandyopadhyay A, Kovaleva V, Carter D, Murphy A, Tasaka M, Raikhel N (2003) The VTI family of SNARE proteins is necessary for plant viability and mediates different protein transport pathways. Plant Cell 15:2885–2899
Tu JX, Fu TD, Zheng YL (1997) Analysis on inheritance and isolocus of the rapeseed GMS 90-2441A (B. napus L.). Journal of Huazhong Agricultural University 16:255–258
Varnier AL, Mazeyrat-Gourbeyre F, Sangwan RS, Clément C (2005) Programmed cell death progressively models the development of anther sporophytic tissues from the tapetum and is triggered in pollen grains during maturation. J Struct Biol 152:118–128
Vitale A, Raikhel NV (1999) What do proteins need to reach different vacuoles? Trends Plant Sci 4:149–155
Vizcay-Barrena G, Wilson Z (2006) Altered tapetal PCD and pollen wall development in the Arabidopsis ms1 mutant. J Exp Bot 57:2709–2717
Wang TW, Balsamo RA, Ratnayake C, Platt KA, Ting JTL, Huang AHC (1997) Identification, subcellular localization, and developmental studies of oleosins in the anther of Brassica napus. Plant J 11:475–487
Wilson ZA, Morroll SM, Dawson J, Swarup R, Tighe PJ (2001) The Arabidopsis MALE STERILITY1 (MS1) gene is a transcriptional regulator of male gametogenesis, with homology to the PHD-finger family of transcription factors. Plant J 28:27–39
Wu HM, Cheung AY (2000) Programmed cell death in plant reproduction. Development 44:267–281
Wu H, Yang M (2005) Reduction in vacuolar volume in the tapetal cells coincides with conclusion of the tetrad stage in Arabidopsis thaliana. Sex Plant Reprod 18:173–178
Zhang W, Sun Y, Timofejeva L, Chen C, Grossniklaus U, Ma H (2006) Regulation of Arabidopsis tapetum development and function by DYSFUNCTIONAL TAPETUM1 (DYT1) encoding a putative bHLH transcription factor. Development 133:3085–3095
Acknowledgements
We thank Dr. Heather A. Owen from the Electron Microscope Laboratory, Department of Biological Sciences in University of Wisconsin for her critical review of the manuscript. This work was financed by funds from National “973” Project (no. 2007CB109006) and National “863” Project (2006AA10Z1B8 and 2009AA101105). We are grateful to Cao Jianbo for the help with the TEM and Qin Lihong for the assistance with the SEM.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Wan, L., Xia, X., Hong, D. et al. Abnormal Vacuolization of the Tapetum During the Tetrad Stage is Associated with Male Sterility in the Recessive Genic Male Sterile Brassica napus L. Line 9012A. J. Plant Biol. 53, 121–133 (2010). https://doi.org/10.1007/s12374-009-9095-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12374-009-9095-x