Abstract
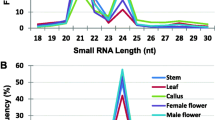
Standardized mechanical planting of single-bud seedcane sett can improve planting efficiency and reduce costs in sugarcane production and is a research focus at present. Small RNA (miRNA) is an important regulator of life activities, which regulates many plant physiological processes such as gene expression, growth, development and metabolism. In this study, miRNAs in six germinating bud samples (S, bud of single-bud sett; D1, upper bud of double-bud sett; D2, lower bud of double-bud sett; T1, upper bud of three-bud sett; T2, median bud of three-bud sett; T3, lower bud of three-bud sett) derived from sugarcane variety GT42 were analyzed. As a result, 8,055,449, 19,172,991, 8,163,579, 11,748,126, 13,547,550 and 12,861,743 clean tags were obtained respectively, and their length was ranged from 16 to 35 nt. For different bud samples, S, D1, D2, T1, T2 and T3, 174, 600, 318, 425, 466, and 317 novel miRNAs were identified respectively, and a total of 1797 miRNAs, 28,042 target genes and 53,141 target sites were obtained from them. As compared with D1, D2, T1, T2 and T3, it was found the sample S showed 219, 157, 108, 44 and 41 up-regulated miRNAs, respectively. Plant hormone signal transduction pathway was the unique pathway enriched by KEGG analysis, and the enriched gene number was 67, 48, 54, 44 and 40 in S_VS_D1, S_VS_D2, S_VS_T1, S_VS_T2 and S_VS_T3, respectively. Furthermore, a total of 19 allelic genes were co-enriched. Among them, the most abundant allelic gene was found to be IAA gene, with a total of 32 gene sequences. All the up-regulated genes were involved in eight aspects metabolic regulation in plant hormone information transduction. The results showed that plant hormone information transduction played a dominant role in the single-bud sett germinating process, which might be the main reason for promoting the early germination and growth. These results can be referred for popularization and application of standardized mechanical planting of single-bud sett in sugarcane.





Similar content being viewed by others
References
Axtell, M.J. 2013. Classification and comparison of small RNAs from plants. Annual Review of Plant Biology 64: 137–159.
Benson, D.A., I. Karsch-Mizrachi, D.J. Lipman, J. Ostell, and E.W. Sayers. 2009. GenBank. Nucleic Acids Research 37 (suppl1): 26–31.
Griffiths-Jones, S., A. Bateman, M. Marshall, A. Khanna, and S.R. Eddy. 2003. Rfam: an RNA family database. Nucleic Acids Research 31 (1): 439–441.
Griffiths-Jones, S., R.J. Grocock, S. van Dongen, A. Bateman, and A.J. Enright. 2006. miRBase: microRNA sequences, targets and gene nomenclature. Nucleic Acids Research 34 (suppl1): 140–144.
Jain, R., S.N. Singh, S. Solomon, and A. Chandra. 2010a. Potential regulatory role of gibberellic acid on sprouting and early stalk growth of sugarcane. Guangxi Agricultural Sciences 41 (9): 1025–1028.
Jain, R., S. Solomon, A.K. Shrivastava, and A. Chandra. 2010b. Sugarcane bud chips: a promising seed material. Sugar Tech 12 (1): 67–69.
Jain, R., S. Solomon, A.K. Shrivastava, and A. Chandra. 2011. Effect of ethephon and calcium chloride on growth and biochemical attributes of sugarcane bud chips. Acta Physiologiae Plantarum 33 (3): 905–910.
Jones-Rhoadeds, M.W., D.P. Bartel, and B. Bartel. 2006. MicroRNAs and their regulatory roles in plants. Annual Review of Plant Biology 57: 19–53.
Kanehisa, M., M. Araki, S. Goto, M. Hattori, M. Hirakawa, M. Itoh, T. Katayama, S. Kawashima, S. Okuda, T. Tokimatsu, and Y. Yamanishi. 2008. KEGG for linking genomes to life and the environment. Nucleic Acids Research 36 (Suppl 1): D480–D484.
Liang, Y.J., X.Q. Zhang, L. Yang, X.H. Liu, L.T. Yang, and Y.R. Li. 2019. Impact of seed coating agents on single-bud seedcane germination and plant growth in commercial sugarcane cultivation. Sugar Tech 21 (3): 383–387.
Lu, X.H., Z.M. Zhu, and J.Y. Yue. 2018. Bioinformatics analysis for high-throughput sequencing of small RNAs in the stigmas of saffron (Crocus sativus). Plant Physiology Journal 54 (5): 827–836.
Luo, Y.W., Z.Q. Qin, T. Liang, Z.Y. Deng, and W.Z. Wang. 2015. Effects on germination and tillering by different drugs coated single bud cane in sugarcane. Agricultural Research and Application 1: 23–25, 29.
Naik, R., S.J.K. Annamalai, N. Vijayan Nair, and N.R. Prasad. 2013. Studies on mechanisation of planting of sugarcane bud chip settlings raised in protrays. Sugar Tech 15 (1): 27–35.
Robin, J.D., A.T. Ludlow, R. LaRanger, W.E. Wright, and J.W. Shay. 2016. Comparison of DNA quantification methods for next generation sequencing. Scientific Reports 6: 24067.
Song, X., Y. Li, X. Cao, and Y. Qi. 2019. MicroRNAs and their regulatory roles in plant-environment interactions. Annual Review of Plant Biology 70: 489–525.
Su, Y.C., Y.Y. Zhang, N. Huang, F. Liu, W.H. Su, L.P. Xu, W. Ahmad, Q.B. Wu, J.L. Guo, and Y.X. Que. 2017. Small RNA sequencing reveals a role for sugarcane miRNAs and their targets in response to Sporisorium scitamineum infection. BMC Genomics 18: 325.
Sunkar, R., and J.K. Zhu. 2004. Novel and stress regulated microRNAs and other small RNAs from Arabidopsis w inside box sign. Plant Cell 16 (8): 2001–2019.
Tan, Q.L., C.N. Li, L.T. Yang, and Y.R. Li. 2013. Cloning and expression analysis of abscisic acid signal transduction key enzyme gene SoSnRK2.1 from sugarcane. Acta Agronomica Sinica 39 (12): 2162–2170.
Vierstra, R.D. 2009. The ubiquitin-26S proteasome system at the nexus of plant biology. Nature Reviews Molecular Cell Biology 10 (6): 385–397.
Wang, R.H., L. Xu, and X.W. Zhu. 2015. Transcriptome-wide characterization of novel and heat-stress responsive microRNAs in radish (Raphanus sativus L.) using next-generation sequencing. Plant Molecular Biology Reporter 33 (4): 867–880.
Wolters, H., and G. Jurgens. 2009. Survival of the flexible: hormonal growth control and adaptation in plant development. Nature Reviews Genetics 10 (5): 305–317.
Wu, K.C., L.P. Wei, C.M. Huang, Y.W. Wei, H.Q. Cao, L. Xu, H.B. Luo, S.L. Jiang, Z.N. Deng, and Y.R. Li. 2018. Transcriptome reveals differentially expressed genes in Saccharum spontaneum GX83-10 leaf under drought stress. Sugar Tech 20 (6): 756–764.
Yan, T., D. Yoo, T.Z. Berardini, L.A. Mueller, D.C. Weems, S. Weng, J.M. Cherry, and S.Y. Rhee. 2005. PatMatch: a program for finding patterns in peptide and nucleotide sequences. Nucleic Acids Research 33 (suppl2): 262–266.
Yang, L., F. Liao, Y.J. Liang, X.H. Liu, L.T. Yang, and Y.R. Li. 2016. Analysis on key influence factors of clean cane seed. Guihaia 36 (3): 267–272.
Zhang, S.F., L. Zhang, J.L. Zhao, L. Zhang, and H.G. Zhang. 2016. Small RNA sequencing and target gene prediction in Larix olgensis. Journal of Beijing Forestry University 38 (12): 64–72.
Acknowledgements
We are grateful to Guangzhou Genedenovo Biotechnology Co., Ltd. for assisting in sequencing and bioinformatics analysis.
Funding
This work was financially supported in part by the Key Project of Science and Technology Innovation Program of Guangxi (Guike 17202005), National Natural Science Foundation of China (31400281, 31760415, 31860593), Fund of Guangxi Academy of Agricultural Sciences (2015YT05), Guangxi Science and Technology Base and Talents Special Project (Guike AD17195100), Fund for Guangxi Innovation Teams of Modern Agriculture Technology (nycytxgxcxtd-03-01).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Wu, KC., Xu, L., Deng, ZN. et al. Small RNA Sequencing Analysis of Germinating Single-bud Seedcane Sett in Sugarcane. Sugar Tech 23, 178–193 (2021). https://doi.org/10.1007/s12355-020-00870-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12355-020-00870-7