Abstract
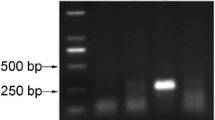
Sugarcane streak mosaic virus (SCSMV, genus Poacevirus) causes sugarcane mosaic disease and leads to considerable economic losses. However, current methods for SCSMV diagnosis are limited. In this study, a novel isothermal assay was established on the basis of reverse transcription–recombinase polymerase amplification (RT-RPA) and evaluated in terms of its ability to detect SCSMV. The established RT-RPA assay with high specificity and reliability was 100-fold more sensitive than the RT-polymerase chain reaction. The evaluation of the temperature limit revealed that the assay had a flexible reaction temperature (26–46 °C), thereby indicating that the reaction mixture can be incubated with simple heating equipment, ambient temperature, or even human body heat. Time limit assessment results revealed that SCSMV can be detected in 5 min at 38 °C and in 20 min at 26 °C, thereby suggesting that the assay was rapid for SCSMV detection. Overall, the RT-RPA assay was specific, sensitive, reliable, and rapid for SCSMV detection, with a flexible and easily reachable reaction temperature. Thus, it can be an ideal method for SCSMV detection in sugarcane.





Similar content being viewed by others
Abbreviations
- CP:
-
Coat protein
- ELISA:
-
Enzyme-linked immunosorbent assay
- IC:
-
Immunocapture
- PCR:
-
Polymerase chain reaction
- RPA:
-
Recombinase polymerase amplification
- RT:
-
Reverse transcription
- SCSMV:
-
Sugarcane streak mosaic virus
- SMD:
-
Sugarcane mosaic disease
- TriMV:
-
Triticum mosaic virus
References
Abd, E.W.A., P. Patel, O. Faye, S. Thaloengsok, D. Heidenreich, P. Matangkasombut, K. Manopwisedjaroen, A. Sakuntabhai, A.A. Sall, and F.T. Hufert. 2015. Recombinase polymerase amplification assay for rapid diagnostics of dengue infection. PLoS ONE 10(6): e0129682.
Amer, H.M., E.W.A. Abd, M.A. Shalaby, F.N. Almajhdi, F.T. Hufert, and M. Weidmann. 2013. A new approach for diagnosis of bovine coronavirus using a reverse transcription recombinase polymerase amplification assay. Journal of Virological Methods 193(2): 337–340.
Babu, B., B.K. Washburn, S.H. Miller, K. Poduch, T. Sarigul, G.W. Knox, F.M. Ochoa-Corona, and M.L. Paret. 2016. A rapid assay for detection of Rose rosette virus using reverse transcription–recombinase polymerase amplification using multiple gene targets. Journal of Virological Methods 240: 78–98.
Bagyalakshmi, K., B. Parameswari, C. Chinnaraja, R. Karuppaiah, V.G. Kumar, and R. Viswanathan. 2012. Genetic variability and potential recombination events in the HC-Pro gene of Sugarcane streak mosaic virus. Archives of Virology 157(7): 1371–1375.
Boyle, D.S., D.A. Lehman, L. Lillis, D. Peterson, M. Singhal, N. Armes, M. Parker, O. Piepenburg, and J. Overbaugh. 2013. Rapid detection of HIV-1 proviral DNA for early infant diagnosis using recombinase polymerase amplification. Mbio 4(2): 49–52.
Chandran, V., and P. Gajjeraman. 2013. A simple precipitation approach for isolation and enrichment of Sugarcane streak mosaic virus. Sugar Tech 15(4): 417–419.
Chatenet, M., C. Mazarin, J.C. Girard, E. Fernandez, D. Gargani, G.P. Rao, M. Royer, B. Lockhart, and P. Rott. 2005. Detection of Sugarcane streak mosaic virus in sugarcane from several Asian countries. Proceedings-International Society of Sugar Cane Technology 25: 656–663.
Compton, J. 1991. Nucleic acid sequence-based amplification. Nature 350(6313): 91–92.
Crannell, Z.A., B. Rohrman, and R. Richards-Kortum. 2014. Equipment-free incubation of recombinase polymerase amplification reactions using body heat. PLoS ONE 9(11): e112146.
Euler, M., Y.J. Wang, D. Heidenreich, P. Patel, O. Strohmeier, S. Hakenberg, M. Niedrig, F.T. Hufert, and M. Weidmann. 2013. Development of a panel of recombinase polymerase amplification assays for detection of biothreat agents. Journal of Clinical Microbiology 51(4): 1110–1117.
Feng, X.Y., L.B. Shen, W.Z. Wang, J.G. Wang, Z.Y. Cao, C.L. Feng, T.T. Zhao, and S.Z. Zhang. 2018. Development of a reverse transcription–recombinase polymerase amplification assay for detection of Sugarcane yellow leaf virus. Sugar Tech. https://doi.org/10.1007/s12355-018-0602-6.
Fu, W.L., S.R. Sun, H.Y. Fu, R.K. Chen, J.W. Su, and S.J. Gao. 2015. A one-step real-time RT-PCR assay for the detection and quantitation of Sugarcane streak mosaic virus. BioMed Research International 2015(2): 1–9.
Gonzales, F.R., M. Castillo, T. Avina, M. Watson, M. Miyano, and M. Longiaru. 1995. Specific detection of HBV DNA in serum by transcription mediated amplification. Hepatology 22(4): 267A.
Gusev, Y., J. Sparkowski, A. Raghunathan, H. Ferguson, J. Montano, N. Bogdan, B. Schweitzer, S. Wiltshire, S.F. Kingsmore, W. Maltzman, and V. Wheeler. 2001. Rolling circle amplification: a new approach to increase sensitivity for immunohistochemistry and flow cytometry. American Journal of Pathology 159(1): 63–69.
He, Z., W.F. Li, R. Yasaka, Y.K. Huang, and Z.X. Zhang. 2014. Molecular variability of Sugarcane streak mosaic virus in China based on an analysis of the P1 and CP protein coding regions. Archives of Virology 159(5): 1149–1154.
Hema, M., N. Kirthi, P. Sreenivasulu, and H.S. Savithri. 2003. Development of recombinant coat protein antibody based IC-RT-PCR for detection and discrimination of Sugarcane streak mosaic virus isolates from Southern India. Archives of Virology 148(6): 1185–1193.
Hema, M., H.S. Savithri, and P. Sreenivasulu. 2001. Antibody and nucleic acid probe-based techniques for detection of Sugarcane streak mosaic virus causing mosaic disease of sugarcane in India. Current Science 81(8): 1105–1108.
Hema, M., P. Sreenivasulu, and H.S. Savithri. 2002. Taxonomic position of Sugarcane streak mosaic virus in the family Potyviridae. Archives of Virology 147(10): 1997–2007.
Kasemsin, P., P. Chiemsombat, and R. Hongprayoon. 2016. Characterization and genetic variation of Sugarcane streak mosaic virus, a Poacevirus infecting sugarcane in Thailand. Modern Applied Science 10(4): 137–149.
Li, W.F., X.Y. Wang, Y.K. Huang, H.L. Shan, R.Y. Zhang, J. Yin, and Z.M. Luo. 2016. Molecular detection techniques of sugarcane important diseases. Plant Protection 42(5): 125–130. (In Chinese).
Liang, S.S., O.J. Alabi, M.B. Damaj, W.L. Fu, S.R. Sun, H.Y. Fu, R.K. Chen, T.E. Mirkov, and S.J. Gao. 2016. Genomic variability and molecular evolution of Asian isolates of Sugarcane streak mosaic virus. Archives of Virology 161(6): 1493–1503.
Londoño, M.A., C.L. Harmon, and J.E. Polston. 2016. Evaluation of recombinase polymerase amplification for detection of begomoviruses by plant diagnostic clinics. Virology Journal 13(1): 1–9.
Mekuria, T.A., S.L. Zhang, and K.C. Eastwell. 2014. Rapid and sensitive detection of Little cherry virus 2 using isothermal reverse transcription–recombinase polymerase amplification. Journal of Virological Methods 205: 24–30.
Notomi, T., H. Okayama, H. Masubuchi, T. Yonekawa, K. Watanabe, N. Amino, and T. Hase. 2000. Loop-mediated isothermal amplification of DNA. Nucleic Acids Research 28(12): e63.
Piepenburg, O., C.H. Williams, D.L. Stemple, and N.A. Armes. 2006. DNA detection using recombination proteins. PLoS Biology 4(7): 1115–1121.
Prabowo, D.B., T. Hadiastono, T. Himawan, and L.K. Putra. 2014. Detection disease of Sugarcane streak mosaic virus (SCSMV) via serological test on sugarcane (Saccharum officinarum L.), weed and insect vector. International Journal of Science and Research 3(1): 88–92.
Putra, L.K., T.H. Astono, S.R.C. Syamsidi, and S. Djauhari. 2015a. Dispersal, yield losses and varietal resistance of Sugarcane streak mosaic virus (SCSMV) in Indonesia. International Journal of Virology 11(1): 32–40.
Putra, L.K., T.H. Astono, S.R.C. Syamsidi, and S. Djauhari. 2015b. Investigation on transmission modes and host range of Sugarcane streak mosaic virus in sugarcane (Saccharum officinarum L.) in Indonesia. Journal of Agricultural and Crop Research 3(4): 59–66.
Putra, L.K., A. Kristini, and E.M.A. Achadian. 2014. Sugarcane streak mosaic virus in Indonesia: distribution, characterisation, yield losses and management approaches. Sugar Tech 16(4): 392–399.
Rao, G.P., M. Chatenet, J.G. Girard, and P. Rott. 2006. Distribution of sugarcane mosaic and sugarcane streak mosaic virus in India. Sugar Tech 8(1): 79–81.
Rosser, A., D. Rollinson, M. Forrest, and B.L. Webster. 2015. Isothermal recombinase polymerase amplification (RPA) of Schistosoma haematobium DNA and oligochromatographic lateral flow detection. Parasites and Vectors 8(1): 446–450.
Silva, G., M. Bömer, C. Nkere, P.L. Kumar, and S.E. Seal. 2015. Rapid and specific detection of Yam mosaic virus by reverse-transcription recombinase polymerase amplification. Journal of Virological Methods 222: 138–144.
Singh, D., and G.P. Rao. 2010. Sudan grass (Sorghum sudanense Stapf): a new Sugarcane streak mosaic virus mechanical host. Guangxi Agricultural Sciences 41(5): 436–438.
Srinivas, K.P., C.V. Subba Reddy, B. Ramesh, P.L. Kumar, and P. Sreenivasulu. 2010. Identification of a virus naturally infecting sorghum in India as Sugarcane streak mosaic virus. European Journal of Plant Pathology 127: 13–19.
Subba Reddy, C.V., P. Sreenivasulu, and G. Sekhar. 2011. Duplex-immunocapture-RT-PCR for detection and discrimination of two distinct potyviruses naturally infecting sugarcane (Saccharum spp. hybrid). Indian Journal of Experimental Biology 49(1): 68–73.
Tatineni, S., F. Qu, R. Li, T.J. Morris, and R. French. 2012. Triticum mosaic poacevirus enlists P1 rather than HC-Pro to suppress RNA silencing-mediated host defense. Virology 433(1): 104–115.
Tomita, N., Y. Mori, H. Kanda, and T. Notomi. 2008. Loop-mediated isothermal amplification (LAMP) of gene sequences and simple visual detection of products. Nature Protocols 3(5): 877–882.
Vincent, M., Y. Xu, and H. Kong. 2004. Helicase-dependent isothermal DNA amplification. EMBO Reports 5(8): 795–800.
Viswanathan, R., M. Balamuralikrishnan, and R. Karuppaiah. 2007. Sugarcane mosaic in India: a cause of combined infection of Sugarcane mosaic virus and Sugarcane streak mosaic virus. Sugar Cane International 25(2): 6–14.
Viswanathan, R., M. Balamuralikrishnan, and R. Karuppaiah. 2008. Characterization and genetic diversity of Sugarcane streak mosaic virus causing mosaic in sugarcane. Virus Genes 36(3): 553–564.
Walker, G.T., M.S. Fraiser, J.L. Schram, M.C. Little, J.G. Nadeau, and D.P. Malinowski. 1992. Strand displacement amplification–an isothermal, in vitro DNA amplification technique. Nucleic Acids Research 20(7): 1691–1696.
Wang, Z.P., H.J. Sun, Q. Guo, S.Q. Xu, J.H. Wang, S.H. Lin, and M.Q. Zhang. 2017. Artificial inoculation method of pokkah boeng disease of sugarcane and screening of resistant germplasm resources in subtropical China. Sugar Tech 19(3): 1–10.
Xie, Y.J., M.Q. Wang, D.L. Xu, R.H. Li, and G.H. Zhou. 2009. Simultaneous detection and identification of four sugarcane viruses by one-step RT-PCR. Journal of Virological Methods 162(1–2): 64–68.
Xu, C., L. Li, W.J. Jin, and Y.S. Wan. 2014. Recombinase polymerase amplification (RPA) of CaMV-35S promoter and nos terminator for rapid detection of genetically modified crops. International Journal of Molecular Sciences 15: 18197–18205.
Zhang, S.L., M. Ravelonandro, P. Russell, N. Mcowen, P. Briard, S. Bohannon, and A. Vrient. 2014. Rapid diagnostic detection of plum pox virus in Prunus plants by isothermal AmplifyRP® using reverse transcription–recombinase polymerase amplification. Journal of Virological Methods 207(3): 114–120.
Zhou, D.G., J.L. Guo, L.Q. Xu, S.W. Gao, Q.L. Lin, Q.B. Wu, L.G. Wu, and Y.X. Que. 2014. Establishment and application of a loop-mediated isothermal amplification (LAMP) system for detection of cry1Ac transgenic sugarcane. Scientific Reports 4(6184): 4912.
Acknowledgements
This study is supported by the National Natural Science Foundation of China (31771865) and the Sugar Crop Research System (CARS-170301).
Author information
Authors and Affiliations
Contributions
SZ-Z conceived and designed the experiments. XY-F performed the experiments, result analysis, and manuscript drafting. LB-S, WZ-W, JG-W, and ZY-C revised the manuscript. All authors have read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Feng, XY., Shen, LB., Wang, WZ. et al. Reverse Transcription–Recombinase Polymerase Amplification Assay for the Detection of Sugarcane Streak Mosaic Virus in Sugarcane. Sugar Tech 21, 645–652 (2019). https://doi.org/10.1007/s12355-018-0675-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12355-018-0675-2