Abstract
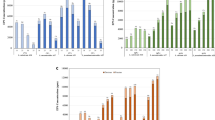
Dextranase was produced from fungus Paecilomyces lilacinus by submerged fermentation under different cultural conditions viz. pH, incubation temperature, inoculum size and days of incubation for maximum enzyme production. Maximum enzyme production was achieved at pH 6.0 of Mandel media and at temperature 30°C. Inoculum size of 1 × 107 spores/ml was found to be optimum for maximum enzyme production. Enzyme production increased with the increase in days of incubation from 3 to 5 days and then declined thereafter. Dextranase units (from 1 to 15 U/100 ml juice) were exogenously added to sugarcane juice with an aim to optimize dextranase application for removal of dextran from cane juice. Addition of dextranase in juice of cane variety CoS 8436, resulted in relatively less increase in dextran content as compared to control during storage. Whereas, dextran content in untreated juice increased 10 times during 24 h of storage, the increase during this period was only 2.3 times in juice treated with 15 U of dextranase. With the application of 1, 2, 5, 10 and 15 units of dextranase in 100 ml of juice, dextran content was decreased by 15.51, 30.82, 50.20, 68.20 and 73.30 % as compared to the control (with no dextranase added) after 24 h of storage. This study finds application in minimizing the dextran problem in sugar industry.
Similar content being viewed by others
References
Abdel-Aziz M S, Fatma, Talkhan N, Janson J C (2007). Purification and characterization of dextranase from a new strain of Penicillium funiculosum. J Appl Sci Res 3: 1509–1516.
Arnold W N, Nguyen T B P, Mann L C (1998). Purification and characterization of a dextranase from Sporothrix schenckii. Arch Microbiol 170: 91–98.
Bhatia S, Jyoti, Uppal S K, Thind K S, Batta S K (2009). Post harvest quality deterioration in sugarcane under different environmental conditions. Sugar Tech 11: 154–160.
Chen J C P, Chou C C (1993). Cane Sugar Hand book. John Wiley and Sons, Inc. New York.
Cheng X, Sun J, Yang J, Chen J, Zhang S (1992). Conditions for dextranase formation by Paecilomyces lilacinus. Wei Sheng Wu Xue Bao 32: 334–339.
Clarke M A, Roberts E J, Goldshell M A, Brannan M A, Carpenter FG, Coll E E (1980). Sucrose loss in manufacture of cane sugar. Sugar Y Azucar 75: 64–68.
Egan BT (1967). Gum content and pH as measures of the losses due to sour storage rot. Proc Queensland Soc. Sugar Cane Technol 35: 31–37.
Eggleston G, Monge A, Montes B, Steward D (2009). Application of dextranases in sugarcane factory: Overcoming practical problems. Sugar Tech 11: 135–141.
Erhardt FA, Stammen S, Jordening HJ (2008). Production, characterization and (Co-) immobilization of dextranase from Penicillium aculeatum. Biotechnol Lett 30: 1069–1073.
Galvez-Mariscal A, Lopez-Munguia A (1991). Production and characterization of a dextranase from an isolated Paecilomyces lilacinus strain. Appl Microbiol Biotechnol 36: 327–331.
Jimenez E R (2009) Dextranase in sugar industry: A review. Sugar Tech 11: 124–134.
Joshi VK, Tamhane DV (1975). Fermentative production of dextranase by Asperigillus luchuensis Inui. Indian Journal of Experimental Biology 13: 55–57.
Kobayashi M, Yokoyama I, Matsuda K (1984). Substrate binding sites of Leuconostoc dextransucrase evaluated by inhibition kinetics. Agri Biol Chem 50: 2585–2590.
Koenig DW, Day DF (1988). Production of dextranase by Lipmyces shrrkeyi. Biotechnology Letters 10: 117–122
Lobanok A G, Zinchenko O N, Shishlo V I (1982). The effect of cultivation conditions and composition of the nutrient medium on dextranse synthesis by the fungus Asperigillus insuetus. Prikl Biokhim Mikrobiol 18: 664–670.
Lowry O H, Rosebrough N J, Farr A L, Randall R J (1951). Protein measurement with Folin phenol reagent. J Biol Chem 193: 265–275.
Madhu, Shukla G L, Prabhu K A (1984). Application of dextranase in the removal of dextran from cane juice. Int Sugar J 86: 136–138.
Mandel M, Anreotti R, Roche (1976). Measurement of saccharifying cellulose. Biotechnol Bioeng Symp 6: 21–23.
Morel du Boil P G, Wienese S (2000). Enzymatic reduction of dextran in process. Laboratory evaluation of dextranases. Procs S Afr Sug Technol Ass 76: 435–443.
Nelson (1944). A photometric adaptation of the Somogyi method for the determination of glucose. J Biol Chem 153: 375–380.
Petrova L I, Molodova G A, Burtseva N N (1975). Conditions of dextranase formation by Penicillium funiculosum 15. Prikl Biokhim Mikrobiol 11: 63–66.
Pleszczynska M, Szczodrak J, Rogalski J, Fiedurek J (1997). Hydrolysis of dextran by Penicllium notatum dextranase and identification of final digeston products. Mycological Research 101: 69–72.
Rauh J S, Cuddihy J A, Falgout R N (1999). Analyzing dextran in sugar industry. A review of dextran in factory and a new analytical technique. Midland Research Laboratories, Inc. Available from URL: http://www.midlandresearchlabsinc.com/doclib/dexrevew.pdf.
Roberts E J, Clarke M A, Goldshall A N (1983). The analysis of dextran in sugar production. Proc. Int. Soc. Sugar Cane Technol 18th Cong. 3: 1374–1383.
Roberts E J, Clarke M A, Goldshell M A, Shoichet A (1984). Dextran analysis in cane juice, syrups and sugars, a new quantity test. J Amer Soc Sugarcane Technol 3:96.
Sharma K P, Rattan B K, Kanwar RS (1989). Improvement in sugar recovery by preventing bacterial polysaccharide production in cane juice. Bhartiva sugar 14: 15–18.
Shimizu E, Unno T, Ohba M, Okoda G (1998). Purification and characterization of an Isomaltotriose-producing endo-dextranase from Fusarium sp. Biosci Biotechnol Biochem 62: 117–122.
Solomon S (1994). Post harvest deterioration of sugarcane: Physical and chemical methods to minimize inversion for higher sugar recovery. Indian J Sugarcane Tech. 9: 27–38.
Solomon S, Ramadurai R, Shanmugnathan S, Shrivastava A K, Deb Santa, Singh I (2003). Management of biological losses in milling tandem to improve sugar recovery. Sugar Tech 5: 137–142.
Solomon S, Shrivastava A K, Yadav R L (2007) Strategies to minimize post harvest sucrose losses in sugarcane: An overview. Proc of 65th Annual Conv. Of STAI: 112–121.
Szczodrak J, Pleszczynska M, Fiedurek (1994) Penicillium notatum I a new source of dextranase. Journal of Industrial Microbiology 13: 315–320.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Bhatia, S., Bhakri, G., Arora, M. et al. Dextranase production from Paecilomyces lilacinus and its application for dextran removal from sugarcane juice. Sugar Tech 12, 133–138 (2010). https://doi.org/10.1007/s12355-010-0026-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12355-010-0026-4