Abstract
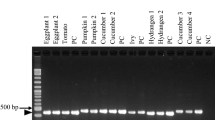
A quick polymerase chain reaction (PCR) assay was developed for the detection of Leifsonia xyli subsp. xyli (Lxx), the bacterial causal agent of ratoon stunting disease (RSD) of sugarcane, in xylem sap samples from stalks. After removal of abiotic impurities and large molecular weight microorganisms by a 3000 rpm, 5 min centrifugation, the Lxx bacteria were precipitated from the xylem sap by a 12,000 rpm, 10 min centrifugation. The Lxx cells were re-suspended in 50 μl freshly prepared Buffer A (0.1M NaOH + 2% Tween 20), lysed by heating at 95°C for 10 min, cooled down on ice for 3 min, and neutralized by mixing with 50 μl of freshly prepared Buffer B (0.1M Tris-HCl, pH 8.0 + 2 mM EDTA). The resulting lysates were directly subjected to conventional PCR with Lxx-specific primers. Of the 31 varieties tested, 19 were positive for Lxx infection, including all FN, GT, and YT varieties. These varieties also tested positive by PCR on DNA templates extracted from xylem sap samples using a CTAB procedure and by two enzyme-immunoassays, dot-blot (DBEIA) and direct antigen coating (DAC-ELISA). DB-EIA and DAC-ELISA detected Lxx in 90.3 and 93.5%, respectively, of the varieties while the detection rate with PCR was 61.3%. The modified PCR assay was quick and more economical. It did not require organic solvent usage or ethanol precipitation, but produced the same level of detection as that of the PCR using DNA samples prepared by the CTAB procedure. All the PCR amplicons were 439 bp in size sharing the same nucleotide sequence. This consensus sequence, GenBank Accession EU723209, aligned perfectly with three other Lxx nucleotide sequences available in the GenBank, AE016822, AF034641, and DQ232616. However, two mis-matches, a (G?A) transversion and a deletion, were found in another nucleotide sequence AF056003.
Similar content being viewed by others
References
Altschul, S.F., Gish, W., Miller, W., Myers, E.W., and Lipman, D.J. (1990). Basic local alignment tool. J. Mol. Biol., 215:403–410.
Astua-Monge, G., Stall, R.E., Davis, M.J. (1995). Diagnosis of ratoon stunting disease of sugarcane by PCR-based procedures. Sugar Azucar, 90:37.
Bailey, R.A. and Bechet, G.R. (1997). Further evidence of the efect of ratoon stunting disease on production under irrigated and rain fed conditions. Page 97–101, In: Proc. 71 Meeting of the South African Sugar Technol. Assoc., Durban and Mount Edgecombe, South Africa.
Benda, G.T.A. and Ricaud, C. (1977). The use of heat treatment for disease control. Proc. Int. Soc. Sugar Cane Technol, 16: 483–496.
Brumbley, S.M., Petrasovits, L.A., Murphy, R.M., Nagel, R.J., Candy, J.M. and Hermann, S.R. (2004). Establishment of a functional genomics platform for Leifsonia xyli subsp. xyli. Mol. Plant-Microbe Interact., 17:175–183.
Chung, C.H., Lin C.P. and Chen C.T. (1994). Development and application of cloned DNA probes for Clavibacter xyli subsp. xyli, the causal agent of sugarcane ratoon stunting. J. Phytopathol., 141:293–301.
Comstock, J.C., Shine, J.M., Davis, M.J. and Dean, J.L. (1996). Relationship between resistance to Clavibacter xyli subsp xyli colonization in sugarcane and spread of ratoon stunting disease in the field. Plant dis., 80:704–708.
Comstock J.C., Irey, M.S., Lockhart, B.E.L. and Wang, Z.K. (1998). Incidence of yellow leaf syndrome in CP cultivars based on polymerase chain reaction and serological techniques. Sugar Cane, 4:21–24.
Croft, B.J., Greet, A.D., Leaman, T.M. and Teakle, D.S. (1994). RSD diagnosis and varietal resistance screening in sugarcane using the EBEIA technique. Austral. Soc. Sugar Cane Technol., 16:143–151.
Croft, B.J. (2002). A method for rating sugarcane cultivars for resistance to ratoon stunting disease based on an enzyme-linked immunoassay. Austral. Plant Pathol., 31:63–66.
Damann, Jr., K.E. (1988). Alkaline-induced metaxylem autofluorescence: A diagnostic symptom of ratoon stunting disease of sugarcane. Phytopathol., 78:233–236.
Damann, K.E. (1992). Effect of sugarcane cultivar susceptibility on spread of ratoon stunting disease by the mechanical harvester. Plant Dis., 76:1148–1149.
Damann, Jr., K.E. and Benda, G.I.A. (1983). Evaluation of commercial heat treatment methods for control of ratoon stunting disease of sugarcane. Plant Dis., 67:966–967.
Damann, K.E. and Hollier, C.A. (1991). Distribution and incidence of ratoon stunting disease in Louisiana Sugarcane. Plant Dis., 75:568–571.
Davis, M. J., Gillaspie, Jr., A.G., Harris, R.W. and Lawson, R.H. (1980). Ratoon stunting disease of sugarcane: Isolation of the causal bacterium. Science, 210:1365–1367.
Davis, M.J., Gillaspie, Jr., A.G., Vidaver, A.K. and Harris, R.W. (1984). Clavibacter, a new genus containing some phytopathogenic coryneform bacteria Clavibacter xyli subsp. xyli sp. nov., subsp. nov. and Clavibacter xyli subsp. cynodontis subsp. nov., pathogens that cause ratoon stunting disease of sugarcane and Bermudagrass stunting disease. Intl. J. Syst. Bacteriol., 34:107–117.
Davis, M.J. (1985). Direct-count techniques for enumerating Clavibacter xyli subsp. xyli which cause ratoon stunting disease of sugarcane. Phytopathol., 75:1226–1231.
Davis, M.J., Rott, P. and Astua-Monge, G. (1998). Nested, multiplex PCR for detection of both Clavibacter xyli subsp. xyli and Xanthomonas albilineans in sugarcane. In/ul: Offered papers abstracts, Vol. 3, 7th Intl. Congress Plant Pathol., Edinburgh, Scotland. 9–16 August 1998. Abstract 3.3.4. Edinburgh, Intl. Soc. Plant Pathol.
Evtushenko L, Dorofeeva L, Subbotin J and Tiedje J. (2000). Leifsonia poae gen. nov., sp. nov., isolated from nematode galls on Poa annua, and reclassification of ‘Corynebacterium aquaticum’ Leifson 1962 as Leifsonia aquatica (ex Leifson 1962) gen. nov., nom. rev., comb. nov. and Clavibacter xyli Davis et al. 1984 with two subspecies as Leifsonia xyli (Davis et al. 1984) gen. nov., comb. nov. Intl. J. Syst. Evol. Microbiol., 50:371–380.
Fegan, M., Croft, B.J., Teakle, D.S., Hayward, A.C. and Smith, G.R. (1998). Sensitive and specific detection of Clavibacter xyli subsp. xyli, causative agent of ratoon stunting disease of sugarcane, with a polymerase chain reaction-based assay. Plant Pathol. 47:495–504.
Gillaspie, Jr., A.G. and Teakle, D.S. (1989). Ratoon stunting disease. Pages 59–80 in: Diseases of Sugarcane — Major Diseases. Ricaud, C., Egan, B.T., Gillaspie Jr., A.G. and Hughes, C.G. (eds). Elsevier, New York.
Grisham, M.P., Pan, Y.-B. and Richard, Jr., E.P. (2007). Early detection of Leifsonia xyli subsp xyli in sugarcane leaves by real-time polymerase chain reaction. Plant Dis., 91:430–434.
Harrison, N.A. and Davis, M.J. (1988). Colonization of vascular tissues by Clavibacter xyli subsp. xyli in stalks of sugarcane cultivars differing in susceptibility to ratoon stunting disease. Phytopathol., 78:722–727.
Hobbs, H.A., Reddy, D.V.R., Rajeswari, R. and Reddy, A.S. (1987). Use of direct antigen coating and protein A coating ELISA procedures for detection of three peanut viruses. Plant Dis., 71:747–749.
Hoy, J.W., Grisham, M.P. and Damann, K.E. (1999). Spread and increase of ratoon stunting disease of sugarcane and comparison of disease detection methods. Plant Dis., 83:1170–1175.
Irvine, J.E. (1976). Factors affecting the expression of juvenile symptoms of the ratoon stunting disease. Proc. Am. Soc. Sugar Cane Technol., 5:109–113.
Monteiro-Vitorello, C.B.M., Camargo, L.E.A., Sluys, M.A.V., Kitajima, J.P., Truffi, D., Amaral, A.M., Harakava, R., Franco, J.C., Wood, D., Oliveira, M.C., Miyaki, C., Takita, M.A., Silva, A.C.R., Furlan, L.R., Carraro, D.M., Camarotte, G., Almeida, N.F., Carrer, H., Coutinho, L.L., El-Dorry, H.A., Ferro, M.I.T., Gagliardi, P.R., Giglioti, E., Goldman, M.H.S., Goldman, G.H., Kimura, E.T., Ferro, E.S., Kuramae, E.E., Lemos, E.G.M., Lemos, M.V.F., Mauro, S.M.Z., Machado, M.A., Marino, C.L., Menck, C.F., Nunes, L.R., Oliveira, R.C., Pereira, G.G., Siqueira, W., Souza, A.A., Tsai, S.M., Zanca, A.S., Simpson, A.J.G., Brumbley, S.M. and Setubal, J.C. (2004). The genome sequence of the Grampositive sugarcane pathogen Leifsonia xyli subsp. xyli. Mol. Plant Microbe Interact., 17:827–836.
Pan, Y.-B., Grisham, M.P., Burner, D.M., Damann, Jr., K.E. and Wei, Q. (1998a). A polymerase chain reaction protocol for the detection of Clavibacter xyli subsp. xyli, the causal bacterium of ratoon stunting disease. Plant Dis., 82:285–290.
Pan, Y.-B., Grisham, M.P., Burner, D.M., Wei, Q. and Damann, Jr., K.E. (1998b). Detecting Clavibacter xyli subsp. xyli by tissue blot DNA hybridization. Sugar Cane, 3:3–8.
Pan, Y.-B., Grisham, M.P. and Wei, Q. (2001). PCR diagnosis of sugarcane leaf scald and ratoon stunting disease. Proc. Intl. Soc. Sugar Cane Technol., 24(II):607–608.
Roach, B.T. (1990). Sampling and diagnostic procedures for testing sugarcane resistance to ratoon stunting disease by phase contrast microscopy. Proc. Austral. Soc. Sugar Cane Technol., 12:111–119.
Shen, W.-K., Zhou, G.-H, Deng, H.-H. and Zhou, L.-Y. (2006). Detection of sugarcane ratoon stunting disease pathogen with polymerase chain reaction (PCR) and nucleotide sequence analysis. Chinese Agricultural Science Bulletin, 22(1):413–416.
Taylor, P.W.J., Petrasovits, L.A., Van der Velde, R., Birch, R.G., Croft, B.J., Fegan, M., Smith, G.R. and Brumbley, S.M. (2003). Development of PCR-based markers for detection of Leifsonia xyli subsp. xyli in fibrovascular fluid of infected sugarcane plants. Austral. Plant Pathol., 32:367–375.
Taylor, P.W.J., Ryan, C.C. and Birch, R.G. (1988). Harvester transmission of leaf scald and ratoon stunting disease. Sugar Cane, 4:11–14.
Young, A.J., Petrasovits, L.A., Croft, B.J., Gillings, M. and Brumbley, S.M. (2006). Genetic uniformity of international isolates of Leifsonia xyli subsp. xyli, causal agent of ratoon stunting disease of sugarcane (Saccharum interspecific hybrids). Australasian Plant Pathol., 35:503–511.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Gao, S.J., Pan, Y.B., Chen, R.K. et al. Quick detection of Leifsonia xyli subsp. xyli by PCR and nucleotide sequence analysis of PCR amplicons from Chinese Leifsonia xyli subsp. xyli isolates. Sugar Tech 10, 334–340 (2008). https://doi.org/10.1007/s12355-008-0059-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12355-008-0059-0