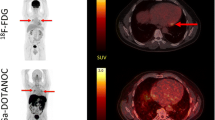
Abstract
Background
Somatostatin receptor is expressed in sarcoid granulomas, and preliminary clinical studies have shown that myocardial sarcoidosis can be identified on somatostatin receptor-targeted PET. We examined the potential clinical use of 68Ga-DOTATATE PET/CT for diagnosis and response assessment in cardiac sarcoidosis compared to 18F-FDG PET/CT.
Methods
Eleven cardiac sarcoidosis patients with 18F-FDG PET/CT were prospectively enrolled for cardiac 68Ga-DOTATATE PET/CT. The two PET/CT studies were interpreted independently and were compared for patient-level and segment-level concordance, as well as for the degree of radiotracer uptake. Follow-up 68Ga-DOTATATE PET/CT was performed in eight patients.
Results
Patient-level concordance was 91%: ten patients had multifocal DOTATATE uptake (active cardiac sarcoidosis) and one patient showed diffuse DOTATATE uptake. Segment-level agreement was 77.1% (Kappa 0.53 ± 0.07). The SUVmax-to-blood pool ratio was lower on 68Ga-DOTATATE PET/CT (3.2 ± 0.6 vs. 4.9 ± 1.5, P = 0.006 on paired t test). Follow-up 68Ga-DOTATATE PET/CT showed one case of complete response and one case of partial response, while 18F-FDG PET/CT showed four cases of response, including three with complete response.
Conclusion
Compared to 18F-FDG PET/CT, 68Ga-DOTATATE PET/CT can identify active cardiac sarcoidosis with high patient-level concordance, but with moderate segment-level concordance, low signal-to-background ratio, and underestimation of treatment response.







Similar content being viewed by others
Abbreviations
- CS:
-
Cardiac sarcoidosis
- PET:
-
Position emission tomography
- 18F-FDG:
-
Fluorine-18-fluorodeoxyglucose
- CMR:
-
Cardiac magnetic resonance imaging
- SSTR:
-
Somatostatin receptor
- 68Ga-DOTATATE:
-
Gallium-68-DOTATATE
- SUV:
-
Standardized uptake value
- CR:
-
Complete response
- PR:
-
Partial response
- NR:
-
No response
- 18F-FLT:
-
Fluorine-18-fluorothymidine
References
Sekhri V, Sanal S, Delorenzo LJ, Aronow WS, Maguire GP. Cardiac sarcoidosis: a comprehensive review. Arch Med Sci 2011;7:546‐54.
Kouranos V, Wells AU, Sharma R. Treatment of cardiac sarcoidosis. Curr Opin Pulm Med 2019;25:519‐25.
Beegle SH, Barba K, Gobunsuy R, Judson MA. Current and emerging pharmacological treatments for sarcoidosis: a review. Drug Des Devel Ther 2013;7:325‐38.
Bravo PE, Singh A, Di Carli MF, Blankstein R. Advanced cardiovascular imaging for the evaluation of cardiac sarcoidosis. J Nucl Cardiol 2019;26:188‐99.
Bravo PE, Raghu G, Rosenthal DG, Elman S, Petek BJ, Soine LA. Risk assessment of patients with clinical manifestations of cardiac sarcoidosis with positron emission tomography and magnetic resonance imaging. Int J Cardiol 2017;241:457‐62.
Youssef G, Leung E, Mylonas I, Nery P, Williams K, Wisenberg G, et al. The use of 18F-FDG PET in the diagnosis of cardiac sarcoidosis: a systematic review and metaanalysis including the Ontario experience. J Nucl Med 2012;53:241‐8.
Ohira H, Tsujino I, Ishimaru S, Oyama N, Takei T, Tsukamoto E, et al. Myocardial imaging with 18F-fluoro-2-deoxyglucose positron emission tomography and magnetic resonance imaging in sarcoidosis. Eur J Nucl Med Mol Imaging 2008;35:933‐41.
Osborne MT, Hulten EA, Murthy VL, Skali H, Taqueti VR, Dorbala S, et al. Patient preparation for cardiac fluorine-18 fluorodeoxyglucose positron emission tomography imaging of inflammation. J Nucl Cardiol 2017;24:86‐99.
ten Bokum AM, Hofland LJ, de Jong G, Bouma J, Melief MJ, Kwekkeboom DJ, et al. Immunohistochemical localization of somatostatin receptor sst2A in sarcoid granulomas. Eur J Clin Invest 1999;29:630‐6.
Bravo PE, Bajaj N, Padera RF, Morgan V, Hainer J, Bibbo CF, et al. Feasibility of somatostatin receptor-targeted imaging for detection of myocardial inflammation: A pilot study. J Nucl Cardiol 2021;28:1089‐99.
Zubin Maslov P, Narula N, Narula J. Somatostatin receptor imaging in active cardiac sarcoidosis: Would less be enough? J Nucl Cardiol 2021;28:1100‐4.
Lee H, Eads JR, Pryma DA. (68) Ga-DOTATATE positron emission tomography-computed tomography quantification predicts response to somatostatin analog therapy in gastroenteropancreatic neuroendocrine tumors. Oncologist 2021;26:21‐9.
Slart R, Glaudemans A, Gheysens O, Lubberink M, Kero T, Dweck MR, et al. Procedural recommendations of cardiac PET/CT imaging: standardization in inflammatory-, infective-, infiltrative-, and innervation- (4Is) related cardiovascular diseases: a joint collaboration of the EACVI and the EANM: summary. Eur Heart J Cardiovasc Imaging 2020;21:1320‐30.
Reiter T, Werner RA, Bauer WR, Lapa C. Detection of cardiac sarcoidosis by macrophage-directed somatostatin receptor 2-based positron emission tomography/computed tomography. Eur Heart J 2015;36:2404.
Passah A, Kaushik P, Patel C, Parakh N. Gallium-68 DOTANOC scan in a patient with suspected cardiac sarcoidosis. J Nucl Cardiol 2018;25:2177‐8.
Lapa C, Reiter T, Li X, Werner RA, Samnick S, Jahns R, et al. Imaging of myocardial inflammation with somatostatin receptor based PET/CT: A comparison to cardiac MRI. Int J Cardiol 2015;194:44‐9.
Slart R, Koopmans KP, van Geel PP, Kramer H, Groen HJM, Gan CT, et al. Somatostatin receptor based hybrid imaging in sarcoidosis. Eur J Hybrid Imaging 2017;1:7.
Lapa C, Reiter T, Kircher M, Schirbel A, Werner RA, Pelzer T, et al. Somatostatin receptor based PET/CT in patients with the suspicion of cardiac sarcoidosis: an initial comparison to cardiac MRI. Oncotarget 2016;7:77807‐14.
Gormsen LC, Haraldsen A, Kramer S, Dias AH, Kim WY, Borghammer P. A dual tracer (68)Ga-DOTANOC PET/CT and (18)F-FDG PET/CT pilot study for detection of cardiac sarcoidosis. EJNMMI Res 2016;6:52.
Pizarro C, Kluenker F, Dabir D, Thomas D, Gaertner FC, Essler M, et al. Cardiovascular magnetic resonance imaging and clinical performance of somatostatin receptor positron emission tomography in cardiac sarcoidosis. ESC Heart Fail 2018;5:249‐61.
Imperiale A, Poindron V, Martinez M, Ohlmann P, Schindler TH, El Ghannudi S. 68Ga-DOTATOC PET for Treatment Efficacy Evaluation of Cardiac Sarcoidosis. Clin Nucl Med 2020;45:e416‐8.
Kolthammer JA, Su KH, Grover A, Narayanan M, Jordan DW, Muzic RF. Performance evaluation of the Ingenuity TF PET/CT scanner with a focus on high count-rate conditions. Phys Med Biol 2014;59:3843‐59.
Cerqueira MD, Weissman NJ, Dilsizian V, Jacobs AK, Kaul S, Laskey WK, et al. Standardized myocardial segmentation and nomenclature for tomographic imaging of the heart. A statement for healthcare professionals from the Cardiac Imaging Committee of the Council on Clinical Cardiology of the American Heart Association. Circulation 2002;105:539‐42.
Itani M, Haq A, Amin M, Mhlanga J, Lenihan D, Iravani A, et al. Myocardial uptake of (68)Ga-DOTATATE: correlation with cardiac disease and risk factors. Acta Radiol 2021:2841851211054193.
Tarkin JM, Joshi FR, Evans NR, Chowdhury MM, Figg NL, Shah AV, et al. Detection of atherosclerotic inflammation by (68)Ga-DOTATATE PET compared to [(18)F]FDG PET imaging. J Am Coll Cardiol 2017;69:1774‐91.
Tarkin JM, Calcagno C, Dweck MR, Evans NR, Chowdhury MM, Gopalan D, et al. (68)Ga-DOTATATE PET identifies residual myocardial inflammation and bone marrow activation after myocardial infarction. J Am Coll Cardiol 2019;73:2489‐91.
Cox CPW, Segbers M, Graven LH, Brabander T, van Assema DME. Standardized image quality for (68)Ga-DOTA-TATE PET/CT. EJNMMI Res 2020;10:27.
Ho SY, Nihoyannopoulos P. Anatomy, echocardiography, and normal right ventricular dimensions. Heart 2006;92:i2-13.
Whitaker J, Rajani R, Chubb H, Gabrawi M, Varela M, Wright M, et al. The role of myocardial wall thickness in atrial arrhythmogenesis. Europace 2016;18:1758‐72.
Nobashi T, Nakamoto Y, Kubo T, Ishimori T, Handa T, Tanizawa K, et al. The utility of PET/CT with (68)Ga-DOTATOC in sarcoidosis: comparison with (67)Ga-scintigraphy. Ann Nucl Med 2016;30:544‐52.
Sharma S, Singh AD, Sharma SK, Tripathi M, Das CJ, Kumar R. Gallium-68 DOTA-NOC PET/CT as an alternate predictor of disease activity in sarcoidosis. Nucl Med Commun 2018;39:768‐78.
Divakaran S, Stewart GC, Lakdawala NK, Padera RF, Zhou W, Desai AS, et al. Diagnostic Accuracy of Advanced Imaging in Cardiac Sarcoidosis. Circ Cardiovasc Imaging 2019;12:e008975.
Zhang J, Li Y, Xu Q, Xu B, Wang H. Cardiac magnetic resonance imaging for diagnosis of cardiac sarcoidosis: A meta-analysis. Can Respir J 2018;2018:7457369.
Pauwels E, Cleeren F, Bormans G, Deroose CM. Somatostatin receptor PET ligands - the next generation for clinical practice. Am J Nucl Med Mol Imaging 2018;8:311‐31.
Norikane T, Yamamoto Y, Maeda Y, Noma T, Dobashi H, Nishiyama Y. Comparative evaluation of (18)F-FLT and (18)F-FDG for detecting cardiac and extra-cardiac thoracic involvement in patients with newly diagnosed sarcoidosis. EJNMMI Res 2017;7:69.
Martineau P, Pelletier-Galarneau M, Juneau D, Leung E, Nery P, deKemp R, et al. FLT-PET for the assessment of systemic sarcoidosis including cardiac and CNS involvement: A prospective study with comparison to FDG-PET. EJNMMI Res 2020;10:154.
Martineau P, Pelletier-Galarneau M, Juneau D, Leung E, Nery PB, de Kemp R, et al. imaging cardiac sarcoidosis with FLT-PET compared with FDG/perfusion-PET: A prospective pilot study. JACC Cardiovasc Imaging 2019;12:2280‐1.
Norikane T, Yamamoto Y, Maeda Y, Noma T, Nishiyama Y. 18F-FLT PET imaging in a patient with sarcoidosis with cardiac involvement. Clin Nucl Med 2015;40:433‐4.
Furuya S, Naya M, Manabe O, Hirata K, Ohira H, Aikawa T, et al. (18)F-FMISO PET/CT detects hypoxic lesions of cardiac and extra-cardiac involvement in patients with sarcoidosis. J Nucl Cardiol 2021;28:2141‐8.
Disclosure
No other potential conflicts of interest relevant to this article exist. No potential conflicts of interest relevant to this article exist for H.L., E.K.S., M.K.V., D.A.P., F.E.M., L.R.G., C.B.C., M.D.R., M.F.D., and P.E.B. D.A.P has provided the following financial interests not relevant to this article: Research Consultant, Five Eleven Pharma Inc.; Research Consultant, Progenics Pharmaceuticals Inc.; Research Consultant, Actinium Pharmaceuticals Inc.; Research Consultant, Ipsen; Research Grant, Siemens AG; Research Grant, Five Eleven Pharma Inc.; Research Grant, Progenics Pharmaceuticals Inc.; and Clinical Trial Funding, Nordic Nanovector ASA.
Author information
Authors and Affiliations
Corresponding author
Additional information
The authors of this article have provided a PowerPoint file, available for download at SpringerLink, which summarizes the contents of the paper and is free for re-use at meetings and presentations. Search for the article DOI on SpringerLink.com.
All editorial decisions for this article, including selection of reviewers and the final decision, were made by guest editor Rob deKemp, PhD.
Funding
The study was funded by the University of Pennsylvania Research Foundation and the Radiological Society of North America (RSD1903). Advanced Accelerator Applications, a Novartis Company, provided 68Ga-DOTATATE for the study.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Lee, H., Schubert, E.K., Vidula, M.K. et al. Potential clinical utility of 68Ga-DOTATATE PET/CT for detection and response assessment in cardiac sarcoidosis. J. Nucl. Cardiol. 30, 1075–1087 (2023). https://doi.org/10.1007/s12350-022-03111-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12350-022-03111-4