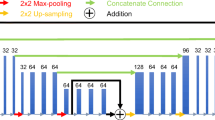
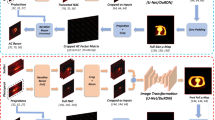
Abstract
It has been proved feasible to generate attenuation maps (μ-maps) from cardiac SPECT using deep learning. However, this assumed that the training and testing datasets were acquired using the same scanner, tracer, and protocol. We investigated a robust generation of CT-derived μ-maps from cardiac SPECT acquired by different scanners, tracers, and protocols from the training data. We first pre-trained a network using 120 studies injected with 99mTc-tetrofosmin acquired from a GE 850 SPECT/CT with 360-degree gantry rotation, which was then fine-tuned and tested using 80 studies injected with 99mTc-sestamibi acquired from a Philips BrightView SPECT/CT with 180-degree gantry rotation. The error between ground-truth and predicted μ-maps by transfer learning was 5.13 ± 7.02%, as compared to 8.24 ± 5.01% by direct transition without fine-tuning and 6.45 ± 5.75% by limited-sample training. The error between ground-truth and reconstructed images with predicted μ-maps by transfer learning was 1.11 ± 1.57%, as compared to 1.72 ± 1.63% by direct transition and 1.68 ± 1.21% by limited-sample training. It is feasible to apply a network pre-trained by a large amount of data from one scanner to data acquired by another scanner using different tracers and protocols, with proper transfer learning.







Similar content being viewed by others
Abbreviations
- MPI:
-
Myocardial perfusion imaging
- SPECT:
-
Single-photon emission computed tomography
- μ-Map:
-
Attenuation maps
- CT:
-
Computed tomography
- AC:
-
Attenuation correction
- DuRDN:
-
Dual squeeze-and-excitation residual dense network
- MLEM:
-
Maximum-likelihood expectation-maximization
References
Danad I, Raijmakers PG, Driessen RS, Leipsic J, Raju R, Naoum C. Comparison of coronary CT angiography, SPECT, PET, and hybrid imaging for diagnosis of ischemic heart disease determined by fractional flow reserve. JAMA Cardiol 2017;2:1100‐7.
Gimelli A, Rossi G, Landi P, Marzullo P, Iervasi G, L’abbate A, et al. Stress/rest myocardial perfusion abnormalities by gated SPECT: still the best predictor of cardiac events in stable ischemic heart disease. J Nucl Med 2009;50:546‐53.
Nishimura T, Nakajima K, Kusuoka H, Yamashina A, Nishimura S. Prognostic study of risk stratification among Japanese patients with ischemic heart disease using gated myocardial perfusion SPECT: J-ACCESS study. Eur J Nucl Med Mol Imaging 2008;35:319‐28.
Patton JA, Turkington TG. SPECT/CT physical principles and attenuation correction. J Nucl Med Technol 2008;36:1‐10.
Tavakoli M, Naij M. Quantitative evaluation of the effect of attenuation correction in SPECT images with CT-derived attenuation. Med Imaging 2019;109485U.
Blankespoor S, Xu X, Kaiki K, Brown J, Tang H, Cann C, et al. Attenuation correction of SPECT using X-ray CT on an emission-transmission CT system: myocardial perfusion assessment. IEEE Trans Nucl Sci 1996;43:2263‐74.
Patchett N, Pawar S, Sverdlov A, Miller E. Does improved technology in spect myocardial perfusion imaging reduce downstream costs? An observational study. Int J Radiol Imaging Technol 2017;3:023.
Rahman MA, Zhu Y, Clarkson E, Kupinski MA, Frey EC, Jha AK. Fisher information analysis of list-mode SPECT emission data for joint estimation of activity and attenuation distribution. Inverse Prob 2020;36:084002.
Sakoshi M, Matsutomo N, Yamamoto T, Sato E. Effect of misregistration between SPECT and CT images on attenuation correction for quantitative bone SPECT imaging. Nihon Hoshasen Gijutsu Gakkai Zasshi 2018;74:452‐8.
Saleki L, Ghafarian P, Bitarafan-Rajabi A, Yaghoobi N, Fallahi B, Ay MR. The influence of misregistration between CT and SPECT images on the accuracy of CT-based attenuation correction of cardiac SPECT/CT imaging: Phantom and clinical studies. Iran J Nucl Med 2019;27:63‐72.
Shi L, Onofrey JA, Liu H, Liu Y-H, Liu C. Deep learning-based attenuation map generation for myocardial perfusion SPECT. Eur J Nucl Med Mol Imaging 2020;47:2383‐95.
Shin H-C, Roth HR, Gao M, Lu L, Xu Z, Nogues I, et al. Deep convolutional neural networks for computer-aided detection: CNN architectures, dataset characteristics and transfer learning. IEEE Trans Med Imaging 2016;35:1285‐98.
Torrey L, Shavlik J, et al. Transfer learning. In: Olivas ES, et al. editors. Handbook of research on machine learning applications and trends: algorithms, methods, and techniques. IGI Global: Hershey; 2010. p. 242‐64.
Liu H, Wu J, Lu W, Onofrey JA, Liu Y-H, Liu C. Noise reduction with cross-tracer and cross-protocol deep transfer learning for low-dose PET. Phys Med Biol 2020;65:185006.
Raghu M, Zhang C, Kleinberg J, Bengio S. Transfusion: Understanding transfer learning for medical imaging. http://arxiv.org/abs/190207208 2019.
Chen X, Zhou B, Shi L, Liu H, Pang Y, Wang R, et al. CT-free attenuation correction for dedicated cardiac SPECT using a 3D dual squeeze-and-excitation residual dense network. J Nucl Cardiol 2021. https://doi.org/10.1007/s12350-021-02672-0.
Ronneberger O, Fischer P, Brox T, et al. U-net: Convolutional networks for biomedical image segmentation. In: Navab N, et al. editors. International conference on medical image computing and computer-assisted intervention. Springern Cham; 2015. p. 234‐41.
Huang G, Liu Z, Van Der Maaten L, Weinberger KQ. Densely connected convolutional networks. In: Proceedings of the IEEE conference on computer vision and pattern recognition; 2017. p. 4700-8.
Hu J, Shen L, Sun G. Squeeze-and-excitation networks. In: Proceedings of the IEEE conference on computer vision and pattern recognition; 2018. p. 7132-41.
Wieczorek H. The image quality of FBP and MLEM reconstruction. Phys Med Biol 2010;55:3161.
Onofrey JA, Casetti-Dinescu DI, Lauritzen AD, Sarkar S, Venkataraman R, Fan RE et al. Generalizable multi-site training and testing of deep neural networks using image normalization. In: 2019 IEEE 16th international symposium on biomedical imaging (ISBI 2019); 2019. p. 348-51.
Paszke A, Gross S, Massa F, Lerer A, Bradbury J, Chanan G, et al. Pytorch: An imperative style, high-performance deep learning library. Adv Neural Inf Process Syst 2019;32:8026‐37.
Smith LN. A disciplined approach to neural network hyper-parameters: Part 1—learning rate, batch size, momentum, and weight decay. http://arxiv.org/abs/180309820 2018.
You K, Long M, Wang J, Jordan MI. How does learning rate decay help modern neural networks? http://arxiv.org/abs/190801878 2019.
Zoccarato O, Marcassa C, Lizio D, Leva L, Lucignani G, Savi A, et al. Differences in polar-map patterns using the novel technologies for myocardial perfusion imaging. J Nucl Cardiol 2017;24:1626‐36.
Gutierrez M, Furuie S, Rebelo M, Moura L, Moro C, Meio C et al. A polar map representation of myocardial kinetic energy from Gated SPECT. In: Proceedings of 18th annual international conference of the IEEE Engineering in Medicine and Biology Society; 1996. p. 660-1.
Nesterov SV, Han C, Mäki M, Kajander S, Naum AG, Helenius H, et al. Myocardial perfusion quantitation with 15 O-labelled water PET: high reproducibility of the new cardiac analysis software (Carimas™). Eur J Nucl Med Mol Imaging 2009;36:1594‐602.
Liu Y-H, Sinusas AJ, Khaimov D, Gebuza BI, Frans JT. New hybrid count-and geometry-based method for quantification of left ventricular volumes and ejection fraction from ECG-gated SPECT: methodology and validation. J Nucl Cardiol 2005;12:55‐65.
Liu Y-H, Sinusas AJ, DeMan P, Zaret BL, Frans J. Quantification of SPECT myocardial perfusion images: methodology and validation of the Yale-CQ method. J Nucl Cardiol 1999;6:190‐203.
Liu Y-H. Quantification of nuclear cardiac images: the Yale approach. J Nucl Cardiol 2007;14:483‐91.
Yang J, Shi L, Wang R, Miller EJ, Sinusas AJ, Liu CJ, et al. Direct attenuation correction using deep learning for cardiac SPECT: A feasibility study. J Nucl Med 2021;62:1645‐52.
Chen X, Zhou B, Xie H, Shi L, Liu H, Holler W, et al. Direct and indirect strategies of deep-learning-based attenuation correction for general purpose and dedicated cardiac SPECT. Eur J Nucl Med Mol Imaging 2022. https://doi.org/10.1007/s00259-022-05718-8.
Acknowledgments
This work is supported by NIH Grants R01HL154345 and R01HL122484.
Author contribution
All authors contribute to the study’s conception and design. Conception and design of this study: XC, BZ, MK, and CL. Data acquisition: XC, HP, KJ, MK, and CL. Network implementation and code writing: XC and BZ. Image reconstruction: XC, HP, and HL. Image analysis: XC, HP, YL, BZ, MK, and CL. Statistical analysis: XC and HL. Clinical evaluations: XC and YL. Writing of the first draft of the manuscript: XC. All authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Data availability
The datasets used in the current study are available from the corresponding author upon reasonable request and with permission of Yale University and University of Massachusetts.
Disclosure
CL, HL, XC, and BZ are named inventors of non-provisional patent applications related to this work that Yale University has filed. HP, KJ, YL, MK have no potential conflicts.
Ethics approval
The retrospective use of the anonymized data in this study was approved by the Institutional Review Boards of Yale University and University of Massachusetts Medical School.
Informed consent
Not applicable.
Author information
Authors and Affiliations
Corresponding authors
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
The authors of this article have provided a PowerPoint file, available for download at SpringerLink, which summarises the contents of the paper and is free for re-use at meetings and presentations. Search for the article DOI on SpringerLink.com.
The authors have also provided an audio summary of the article, which is available to download as ESM, or to listen to via the JNC/ASNC Podcast.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Chen, X., Hendrik Pretorius, P., Zhou, B. et al. Cross-vender, cross-tracer, and cross-protocol deep transfer learning for attenuation map generation of cardiac SPECT. J. Nucl. Cardiol. 29, 3379–3391 (2022). https://doi.org/10.1007/s12350-022-02978-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12350-022-02978-7