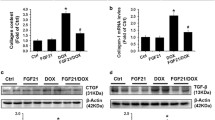
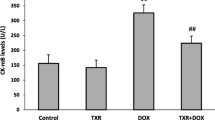
Abstract
Background
Oxidative stress and its interference on myocardial metabolism play a major role in Doxorubicin (DXR) cardiotoxic cascade.
Methods
Mice models of neuroblastoma (NB) were treated with 5 mg DXR/kg, either free (Free-DXR) or encapsulated in untargeted (SL[DXR]) or in NB-targeting Stealth Liposomes (pep-SL[DXR] and TP-pep-SL[DXR]). Control mice received saline. FDG-PET was performed at baseline (PET1) and 7 days after therapy (PET2). At PET2 Troponin-I and NT-proBNP were assessed. Explanted hearts underwent biochemical, histological, and immunohistochemical analyses. Finally, FDG uptake and glucose consumption were simultaneously measured in cultured H9c2 in the presence/absence of Free-DXR (1 μM).
Results
Free-DXR significantly enhanced the myocardial oxidative stress. Myocardial-SUV remained relatively stable in controls and mice treated with liposomal formulations, while it significantly increased at PET2 with respect to baseline in Free-DXR. At this timepoint, myocardial-SUV was directly correlated with both myocardial redox stress and hexose-6-phosphate-dehydrogenase (H6PD) enzymatic activity, which selectively sustain cellular anti-oxidant mechanisms. Intriguingly, in vitro, Free-DXR selectively increased FDG extraction fraction without altering the corresponding value for glucose.
Conclusion
The direct correlation between cardiac FDG uptake and oxidative stress indexes supports the potential role of FDG-PET as an early biomarker of DXR oxidative damage.






Similar content being viewed by others
Abbreviations
- DXR:
-
Doxorubicin
- Free-DXR:
-
Free Doxorubicin
- SL[DXR]:
-
Doxorubicin-loaded stealth liposome (Caelyx)
- pep-SL[DXR]:
-
YSHSHSYWLRSGGGC peptide-targeted, Doxorubicin-loaded stealth liposome
- TP-pep-SL[DXR]:
-
CRALKYSHSHSYWLRSGGG peptide-targeted, Doxorubicin-loaded stealth liposome
- H6PD:
-
Hexose-6-phosphate-dehydrogenase
- HK:
-
Hexokinase
- PFK:
-
Phosphofructokinase
- G6PD:
-
Glucose-6-phosphate-dehydrogenase
- G6Pase:
-
Glucose-6-phosphate-phosphatase
- GR:
-
Glutathione reductase
- MDA:
-
Malondialdehyde
- GSH:
-
Reduced glutathione
- ROS:
-
Reactive oxygen species
- H2DCFDA:
-
2′,7′-Dichlorofluorescein diacetate
- Trx:
-
Thioredoxin
References
Chen MH, Colan SD, Diller L. Cardiovascular disease: Cause of morbidity and mortality in adult survivors of childhood cancers. Circ Res. 2011;108:619–28.
Octavia Y, Tocchetti CG, Gabrielson KL, Janssens S, Crijns HJ, Moens AL. Doxorubicin-induced cardiomyopathy: From molecular mechanisms to therapeutic strategies. J Mol Cell Cardiol. 2012;52:1213–25.
Carvalho RA, Sousa RP, Cadete VJ, Lopaschuk GD, Palmeira CM, Bjork JA, et al. Metabolic remodeling associated with subchronic doxorubicin cardiomyopathy. Toxicology. 2010;270:92–8.
Bauckneht M, Ferrarazzo G, Fiz F, Morbelli S, Sarocchi M, Pastorino F, et al. Doxorubicin effect on myocardial metabolism as a pre-requisite for subsequent development of cardiac toxicity: A translational 18F-FDG PET/CT observation. J Nucl Med. 2017;58:1638–45.
Bauckneht M, Morbelli S, Fiz F, Ferrarazzo G, Piva R, Nieri A, et al. A score-based approach to 18F-FDG PET images as a tool to describe metabolic predictors of myocardial doxorubicin susceptibility. Diagnostics (Basel). 2017;26:7.
Sarocchi M, Bauckneht M, Arboscello E, Capitanio S, Marini C, Morbelli S, et al. An increase in myocardial 18-fluorodeoxyglucose uptake is associated with left ventricular ejection fraction decline in Hodgkin lymphoma patients treated with anthracycline. J Transl Med. 2018;16:295.
Gorla AK, Sood A, Prakash G, Parmar M, Mittal BR. Substantial increase in myocardial FDG uptake on interim PET/CT may be an early sign of adriamycin-induced cardiotoxicity. Clin Nucl Med. 2016;41:462–3.
Marini C, Ravera S, Buschiazzo A, Bianchi G, Orengo AM, Bruno S, et al. Discovery of a novel glucose metabolism in cancer: the role of endoplasmic reticulum beyond glycolysis and pentose phosphate shunt. Sci Rep. 2016;6:25092.
Clarke JL, Mason PJ. Murine hexose-6-phosphate dehydrogenase: a bifunctional enzyme with broad substrate specificity and 6-phosphogluconolactonase activity. Arch Biochem Biophys. 2003;415:229–34.
Bublitz C, Steavenson S. The pentose phosphate pathway in the endoplasmic reticulum. J Biol Chem. 1988;263:12849–53.
Tsachaki M, Mladenovic N, Štambergová H, Birk J, Odermatt A. Hexose-6-phosphate dehydrogenase controls cancer cell proliferation and migration through pleiotropic effects on the unfolded-protein response, calcium homeostasis, and redox balance. FASEB J 2018;8:fj201700870RR.
Rogoff D, Black K, McMillan DR, White PC. Contribution of hexose-6-phosphate dehydrogenase to NADPH content and redox environment in the endoplasmic reticulum. Redox Rep. 2010;15:64–70.
Cossu I, Bottoni G, Loi M, Emionite L, Bartolini A, Di Paolo D, et al. Neuroblastoma-targeted nanocarriers improve drug delivery and penetration, delay tumor growth and abrogate metastatic diffusion. Biomaterials. 2015;68:89–99.
Ponzoni M, Curnis F, Brignole C, Bruno S, Guarnieri D, Sitia L, et al. Enhancement of tumour homing by chemotherapy-loaded nanoparticles. Small. 2018;14:e1802886.
Buschiazzo A, Cossu V, Bauckneht M, Orengo A, Piccioli P, Emionite L, et al. Effect of starvation on brain glucose metabolism and 18F-2-fluoro-2-deoxyglucose uptake: an experimental in vivo and ex-vivo study. EJNMMI Res. 2018;8:44.
Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–54.
Ravera S, Bartolucci M, Cuccarolo P, Litamè E, Illarcio M, Calzia D, et al. Oxidative stress in myelin sheath: The other face of the extramitochondrial oxidative phosphorylation ability. Free Radic Res. 2015;49:1156–64.
Castellani P, Angelini G, Delfino L, Matucci A, Rubartelli A. The thiol redox state of lymphoid organs is modified by immunization: role of different immune cell populations. Eur J Immunol. 2008;38:2419–25.
Vené R, Delfino L, Castellani P, Balza E, Bertolotti M, Sitia R, et al. Redox remodeling allows and controls B cell activation and differentiation. Antioxid Redox Signal. 2010;13:1145–55.
Scussolini M, Bauckneht M, Cossu V, Bruno S, Orengo A, Piccioli P, et al. G6Pase location in the endoplasmic reticulum: Implications on compartmental analysis of FDG uptake in cancer cells. Sci Rep 2019 (in press).
Rosner B. Fundamentals of Biostatistics. 7th ed. Boston: Brooks/Cole; 2011.
Paranka NS, Dorr RT. Effect of doxorubicin on glutathione and glutathione-dependent enzymes in cultured rat heart cells. Anticancer Res. 1994;14:2047–52.
Li L, Pan Q, Han W, Liu Z, Li L, Hu X. Schisandrin B prevents doxorubicin-induced cardiotoxicity via enhancing glutathione redox cycling. Clin Cancer Res. 2007;13:6753–60.
Hrelia S, Fiorentini D, Maraldi T, Angeloni C, Bordoni A, Biagi PL, et al. Doxorubicin induces early lipid peroxidation associated with changes in glucose transport in cultured cardiomyocytes. Biochim Biophys Acta. 2002;1567:150–6.
Dhingra R, Margulets V, Chowdhury SR, Thliveris J, Jassal D, Fernyhough P, et al. Bnip3 mediates doxorubicin-induced cardiac myocyte necrosis and mortality through changes in mitochondrial signaling. Proc Natl Acad Sci USA. 2014;111:E5537–44.
Herman EH, Lipshultz SE, Rifai N, Zhang J, Papoian T, Yu ZX, et al. Use of cardiac troponin T levels as an indicator of doxorubicin-induced cardiotoxicity. Cancer Res. 1998;58:195–7.
Zhong M, Alonso CE, Taegtmeyer H, Kundu BK. Quantitative PET imaging detects early metabolic remodeling in a mouse model of pressure-overload left ventricular hypertrophy in vivo. J Nucl Med. 2013;54:609–15.
Rahman A, Carmichael D, Harris M, Roh JK. Comparative pharmacokinetics of free doxorubicin and doxorubicin entrapped in cardiolipin liposomes. Cancer Res. 1986;46:2295–9.
Dávila-Román VG, Vedala G, Herrero P, de las Fuentes L, Rogers JG, Kelly DP, et al. Altered myocardial fatty acid and glucose metabolism in idiopathic dilated cardiomyopathy. J Am Coll Cardiol 2002;40:271e7.
Depre C, Vanoverschelde JL, Taegtmeyer H. Glucose for the heart. Circulation. 1999;99:578–88.
Sokoloff L, Reivich M, Kennedy C, Des Rosiers MH, Patlak CS, Pettigrew KD, et al. The [14C]deoxyglucose method for the measurement of local cerebral glucose utilization: Theory, procedure, and normal values in the conscious and anesthetized albino rat. J Neurochem. 1977;28:897–916.
Bøtker HE, Goodwin GW, Holden JE, Doenst T, Gjedde A, Taegtmeyer H. Myocardial glucose uptake measured with fluorodeoxyglucose: A proposed method to account for variable lumped constants. J Nucl Med. 1999;40:1186–96.
Jung KH, Lee JH, Thien Quach CH, Paik JY, Oh H, Park JW, et al. Resveratrol suppressed cancer cell glucose uptake by targeting reactive oxygen species-mediated hypoxia-inducible factor-1α activation. J Nucl Med. 2013;54:2161–7.
Chen L, Zhou Y, Tang X, Yang C, Tian Y, Xie R, et al. EGFR mutation decreases FDG uptake in non-small cell lung cancer via the NOX4/ROS/GLUT1 axis. Int J Oncol. 2019;54:370–80.
Wang G, Li Y, Yang Z, Xu W, Yang Y, Tan X. ROS mediated EGFR/MEK/ERK/HIF-1α loop regulates glucose metabolism in pancreatic cancer. Biochem Biophys Res Commun. 2018;500:873–8.
Sen S, Kundu BK, Wu HC, Hashmi SS, Guthrie P, Locke LW, et al. Glucose regulation of load-induced mTOR signaling and ER stress in mammalian heart. J Am Heart Assoc. 2013;2:e004796.
Disclosure
The authors have indicated that they have no financial conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
The authors of this article have provided a PowerPoint file, available for download at SpringerLink, which summarises the contents of the paper and is free for re-use at meetings and presentations. Search for the article DOI on SpringerLink.com.
Funding
This study was partially funded by the program “Ricerca Corrente” to AR and line “Guest-Cancer Interactions” by Compagnia di San Paolo (to CM, project ID Prot.: 2015.AAI4110.U4917), Italian Ministry of Heath (EuroNanoMed II-2015 to FP and “Cinque per mille” 2014 and 2015 to AR) and Italian Association for Cancer Research (AIRC grants IG 14231 and 18474 to MP; IG 15434 to AR) and by Telethon to PP.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Bauckneht, M., Pastorino, F., Castellani, P. et al. Increased myocardial 18F-FDG uptake as a marker of Doxorubicin-induced oxidative stress. J. Nucl. Cardiol. 27, 2183–2194 (2020). https://doi.org/10.1007/s12350-019-01618-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12350-019-01618-x