Abstract
Background
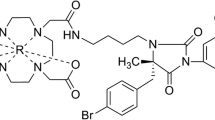
Chemokine receptor 5 (CCR5) plays an important role in atherosclerosis. Our objective was to develop a SPECT tracer targeting CCR5 for imaging plaque inflammation by radiolabeling D-Ala-peptide T-amide (DAPTA), a CCR5 antagonist, with 111In.
Methods
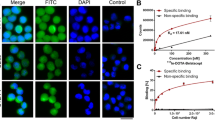
1,4,7,10-tetraazacyclododecane-1,4,7,10-tetraacetic acid (DOTA) conjugated DAPTA (DOTA-DAPTA) was labeled with 111In. Cell uptake studies were conducted in U87-CD4-CCR5 and U87-MG cells. Biodistribution was determined in C57BL/6 mice. Autoradiography, en face and Oil Red O (ORO) imaging studies were performed in ApoE−/− mice.
Results
DOTA-DAPTA was radiolabeled with 111In with high radiochemical purity (> 98%) and specific activity (70 MBq·nmol). 111In-DOTA-DAPTA exhibited fast blood and renal clearance and high spleen uptake. The U87-CD4-CCR5 cells had significantly higher uptake in comparison to the U87-MG cells. The cell uptake was reduced by three times with DAPTA, indicating the receptor specificity of the uptake. Autoradiographic images showed significantly higher lesion uptake of 111In-DOTA-DAPTA in ApoE−/− mice than that in C57BL/6 mice. The tracer uptake in 4 month old ApoE−/− high fat diet (HFD) mice with blocking agent was twofold lower than the same mice without the blocking agent, demonstrating the specificity of the tracer for the CCR5 receptor.
Conclusion
111In-DOTA-DAPTA, specifically targeting chemokine receptor CCR5, is a potential SPECT agent for imaging inflammation in atherosclerosis.






Similar content being viewed by others
Abbreviations
- CCR5:
-
Chemokine receptor 5
- SPECT:
-
Single photon emission computed tomography
- DAPTA:
-
d-Ala-peptide T-amide
- DOTA:
-
1,4,7,10-tetraazacyclododecane-1,4,7,10-tetraacetic acid
- HPLC:
-
High performance liquid chromatography
- ApoE−/− :
-
Apolipoprotein E knock-out
- HFD:
-
High fat diet
- %ID·g:
-
Percent injected dose per gram
- p.i.:
-
Post injection
- ORO:
-
Oil Red O
References
Weber C, Noels H. Atherosclerosis: Current pathogenesis and therapeutic options. Nat Med. 2011;17:1410-22.
Koenen RR, Weber C. Therapeutic targeting of chemokine interactions in atherosclerosis. Nat Rev Drug Discov. 2010;9:141-53.
Braunersreuther V, Mach F, Steffens S. The specific role of chemokines in atherosclerosis. Thromb Haemost. 2007;97:714-21.
Charo IF, Ransohoff RM. The many roles of chemokines and chemokine receptors in inflammation. N Engl J Med. 2006;354:610-21.
Rudd JH, Warburton EA, Fryer TD, Jones HA, Clark JC, Antoun N, et al. Imaging atherosclerotic plaque inflammation with [18F]-fluorodeoxyglucose positron emission tomography. Circulation. 2002;105:2708-11.
Rudd JH, Hyafil F, Fayad ZA. Inflammation imaging in atherosclerosis. Arterioscler Thromb Vasc Biol. 2009;29:1009-16.
Quillard T, Libby P. Molecular imaging of atherosclerosis for improving diagnostic and therapeutic development. Circ Res. 2012;111:231-44.
Jones KL, Maguire JJ, Davenport AP. Chemokine receptor CCR5: From AIDS to atherosclerosis. Br J Pharmacol. 2011;162:1453-69.
Tacke F, Alvarez D, Kaplan TJ, Jakubzick C, Spanbroek R, Llodra J, et al. Monocyte subsets differentially employ CCR2, CCR5, and CX3CR1 to accumulate within atherosclerotic plaques. J Clin Invest. 2007;117:185-94.
Quinones MP, Martinez HG, Jimenez F, Estrada CA, Dudley M, Willmon O, et al. CC chemokine receptor 5 influences late-stage atherosclerosis. Atherosclerosis. 2007;195:e92-103.
Luehmann HP, Pressly ED, Detering L, Wang C, Pierce R, Woodard PK, et al. PET/CT imaging of chemokine receptor CCR5 in vascular injury model using targeted nanoparticles. J Nucl Med. 2014;55:629-34.
Ruff MR, Polianova M, Yang Q, Leoung GS, Ruscetti FW, Pert CB. Update on D-Ala-Peptide T-Amide (DAPTA): A viral entry inhibitor that blocks CCR5 chemokine receptors. Curr HIV Res. 2003;1:51-67.
Rosi S, Pert CB, Ruff MR, McGann-Gramling K, Wenk GL. Chemokine receptor 5 antagonist D-Ala-peptide T-amide reduces microglia and astrocyte activation within the hippocampus in a neuroinflammatory rat model of Alzheimer’s disease. Neuroscience. 2005;134:671-6.
Polianova MT, Ruscetti FW, Pert CB, Ruff MR. Chemokine receptor-5 (CCR5) is a receptor for the HIV entry inhibitor peptide T (DAPTA). Antiviral Res. 2005;67:83-92.
Prior P, Timmins R, Petryk J, Strydhorst J, Duan Y, Wei L, et al. A modified TEW approach to scatter correction for In-111 and Tc-99m dual-isotope small-animal SPECT. Med Phys. 2016;43:5503-13.
Caobelli F, Wollenweber T, Bavendiek U, Kuhn C, Schutze C, Geworski L, et al. Simultaneous dual-isotope solid-state detector SPECT for improved tracking of white blood cells in suspected endocarditis. Eur Heart J. 2016;38:436-43.
Slomka PJ, Berman DS, Germano G. New cardiac cameras: Single-photon emission CT and PET. Semin Nucl Med. 2014;44:232-51.
Zhao Y, Kuge Y, Zhao S, Morita K, Inubushi M, Strauss HW, et al. Comparison of 99mTc-annexin A5 with 18F-FDG for the detection of atherosclerosis in ApoE−/− mice. Eur J Nucl Med Mol Imaging. 2007;34:1747-55.
Kamkar M, Wei L, Gaudet C, Bugden M, Petryk J, Duan Y, et al. Evaluation of apoptosis with 99mTc-rhAnnexin V-128 and inflammation with 18F-FDG in a low-dose irradiation model of atherosclerosis in apolipoprotein E-deficient mice. J Nucl Med. 2016;57:1784-91.
Agool A, Glaudemans AW, Boersma HH, Dierckx RA, Vellenga E, Slart RH. Radionuclide imaging of bone marrow disorders. Eur J Nucl Med Mol Imaging. 2011;38:166-78.
Liu Y, Abendschein D, Woodard GE, Rossin R, McCommis K, Zheng J, et al. Molecular imaging of atherosclerotic plaque with 64Cu-labeled natriuretic peptide and PET. J Nucl Med. 2010;51:85-91.
Liu Y, Pierce R, Luehmann HP, Sharp TL, Welch MJ. PET imaging of chemokine receptors in vascular injury-accelerated atherosclerosis. J Nucl Med. 2013;54:1135-41.
Whitman SC. A practical approach to using mice in atherosclerosis research. Clin Biochem Rev. 2004;25:81-93.
Chen G, Roy I, Yang C, Prasad PN. Nanochemistry and nanomedicine for nanoparticle-based diagnostics and therapy. Chem Rev. 2016;116:2826-85.
Duan Y, Wei L, Petryk J, Ruddy TD. Formulation, characterization and tissue distribution of a novel pH-sensitive long-circulating liposome-based theranostic suitable for molecular imaging and drug delivery. Int J Nanomedicine. 2016;11:5697-708.
Getz GS, Reardon CA. Animal models of atherosclerosis. Arterioscler Thromb Vasc Biol. 2012;32:1104-15.
Acknowledgements
This study was funded in part by the Ontario Research Fund (ORF RE07-021). Dr Wei Gan was supported through Mitacs Elevate Postdoctoral Fellowship. We are very grateful for the technical support from Animal Care technicians.
Authorship
Lihui Wei and Terrence Ruddy proposed the study objectives and designed the experiments. Lihui Wei, Wei Gan and Yin Duan performed the chemistry and radiochemistry synthesis and characterization experiments. Julia Petryk and Chantal Gaudet conducted the animal experiments. Lihui Wei and Maryam Kamkar performed the cell uptake studies. Lihui Wei and Terrence Ruddy analyzed the data and drafted the paper. All authors reviewed and provided comments for revising the paper.
Disclosure
Dr. Terrence Ruddy has received research grants from GE HealthCare and Advanced Accelerator Applications. Dr. Lihui Wei is a full-time employee of Nordion Inc. All other authors declare that they have no conflict of interest.
Research Involving Human Participants and/or Animals
The care and use of animals were conducted in compliance with the guidelines of the Canadian Council on Animal Care and with approval from the Animal Care Committee at the University of Ottawa. The procedures performed for this study did not involve human participants.
Author information
Authors and Affiliations
Corresponding author
Additional information
The authors of this article have provided a PowerPoint file, available for download at SpringerLink, which summarises the contents of the paper and is free for re-use at meetings and presentations. Search for the article DOI on SpringerLink.com.
Electronic Supplementary Material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Wei, L., Petryk, J., Gaudet, C. et al. Development of an inflammation imaging tracer, 111In-DOTA-DAPTA, targeting chemokine receptor CCR5 and preliminary evaluation in an ApoE−/− atherosclerosis mouse model. J. Nucl. Cardiol. 26, 1169–1178 (2019). https://doi.org/10.1007/s12350-018-1203-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12350-018-1203-1