Abstract
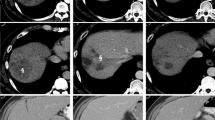
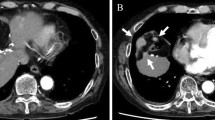
A man in his eighties presented with a history of bilateral leg congestive phlebitis, and multiple hepatocellular carcinoma (HCC) treated with sorafenib. When the dose was increased to 400 mg, ulcers appeared under both knees, which worsened, and the drug was discontinued 2 months after administration. However, the ulcers to 30 mm in diameter, requiring debridement and antibiotics. The HCC showed a complete response (CR) based on modified-RECIST criteria; however, after several rounds of locoregional therapy for recurrence, multiple HCCs and metastatic lesions in the Morrison’s fossa were detected. Therefore, atezolizumab 1200 mg–bevacizumab 900 mg was started. After the first course, the patient complained of pain below both knees, and when the second course was administered, leg ulcers re-appeared and rapidly worsened. The ulcers were circular and multiple and progressed to deep digging, leading to tendon exposure. Bevacizumab-induced congestive venous ulcer was diagnosed, requiring skin grafts to heal. HCC then showed a CR based on m-RECIST criteria. Initially, the cause of the ulcer was thought to be immune-related adverse effects due to atezolizumab, but experience with sorafenib led us to conclude that the cause was stagnant venous ulcers due to vascular endothelial growth factor receptor inhibitor, which inhibited angiogenesis.





Similar content being viewed by others
References
Llovet JM, Ricci S, Mazzaferro V, et al. Sorafenib in advanced hepatocellular carcinoma. N Engl J Med. 2008;359:378–90.
Hurwitz H, Fehrenbacher L, Novotny W, et al. Bevacizumab plus irinotecan, fluorouracil, and leucovorin for metastatic colorectal cancer. N Engl J Med. 2004;350:2335–42.
Finn RS, Qin S, Ikeda M, et al. Atezolizumab plus bevacizumab in unresectable hepatocellular carcinoma. N Engl J Med. 2020;382:1894–905.
Llovet JM, Kelley RK, Villanueva A, et al. Hepatocellular carcinoma. Nat Rev Dis Primers. 2021;7:6.
Cheng AL, Qin S, Ikeda M, et al. Updated efficacy and safety data from IMbrave150: atezolizumab plus bevacizumab vs. sorafenib for unresectable hepatocellular carcinoma. J Hepatol. 2022;76:862–73.
Scappaticci FA, Fehrenbacher L, Cartwright T, et al. Surgical wound healing complications in metastatic colorectal cancer patients treated with bevacizumab. J Surg Oncol. 2005;91:173–80.
Hayashi H, Sawada K, Hasebe T, et al. A successful case of hepatocellular carcinoma treated with atezolizumab plus bevacizumab with multisystem immune-related adverse events. Intern Med. 2022;61:3497–502.
Kichenadasse G, Miners JO, Mangoni AA, et al. Multiorgan immune-related adverse events during treatment with atezolizumab. J Natl Compr Canc Netw. 2020;18:1191–9.
Miyakis S, Lockshin MD, Atsumi T, et al. International consensus statement on an update of the classification criteria for definite antiphospholipid syndrome (APS). J Thromb Haemost. 2006;4:295–306.
Wilson WA, Gharavi AE, Koike T, et al. International consensus statement on preliminary classification criteria for definite antiphospholipid syndrome: report of an international workshop. Arthritis Rheum. 1999;42:1309–11.
Zhang L, Zhou Q, Ma L, et al. Meta-analysis of dermatological toxicities associated with sorafenib. Clin Exp Dermatol. 2011;36:344–50.
Lacouture ME, Wu S, Robert C, et al. Evolving strategies for the management of hand-foot skin reaction associated with the multitargeted kinase inhibitors sorafenib and sunitinib. Oncologist. 2008;13:1001–11.
Gomez P, Lacouture ME. Clinical presentation and management of hand-foot skin reaction associated with sorafenib in combination with cytotoxic chemotherapy: experience in breast cancer. Oncologist. 2011;16:1508–19.
Stieb S, Riesterer O, Brüssow C, et al. Radiation recall dermatitis induced by sorafenib : a case study and review of the literature. Strahlenther Onkol. 2016;192:342–8.
Mehta K, Kaubisch A, Tang J, et al. Radiation recall dermatitis in patients treated with sorafenib. Case Rep Oncol Med. 2018;2018:2171062.
Oh D, Park HC, Lim HY, et al. Sorafenib-triggered radiation recall dermatitis with a disseminated exanthematous reaction. Radiat Oncol J. 2013;31:171–4.
Robbins J, Wollner I, Ryu S. Sorafenib induced radiation recall dermatitis after spine radiosurgery. J Radiosurg SBRT. 2011;1:71–4.
Sirka CS, Sahu K, Pradhan S, et al. Sorafenib-induced grade III hand-foot skin reaction with ulcerative dermatitis on scrotum, penis, and earlobe. Indian J Dermatol Venereol Leprol. 2019;85:623–6.
Liu KC, Hao YH, Lv WF, et al. Transarterial chemoembolization combined with sorafenib in patients with BCLC stage C hepatocellular carcinoma. Drug Des Devel Ther. 2020;14:3461–8.
Mantovani A, Álvares-Da-Silva MR. Anaphylaxis preceded by erythema multiforme with sorafenib: first case report. Ann Hepatol. 2019;18:777–9.
Dohmen K. Severe ulcerative skin lesions due to lenvatinib. Clin Gastroenterol Hepatol. 2020;18:e113.
Chugai Pharmaceutical Co., Avastin®. Summary of Product Characteristics, 2020. https://www.kegg.jp/medicus-bin/japic_med?japic_code=00052871. Accessed 1 Oct 2022.
Erinjeri JP, Fong AJ, Kemeny NE, et al. Timing of administration of bevacizumab chemotherapy affects wound healing after chest wall port placement. Cancer. 2011;117:1296–301.
Suehara Y, Osawa H, Kubota D, et al. Large skin ulcer due to a subcutaneous orthopaedic implant after bevacizumab therapy: a case report. JBJS Case Connect. 2016;6: e70.
Vila-Payeras A, Iglesias-González M, Terrasa-Sagristá F, et al. Cutaneous ulcer with thrombogenic vasculopathy in a patient receiving bevacizumab. Indian J Dermatol Venereol Leprol. 2021;87:268–70.
Ahn JW, Shalabi D, Correa-Selm LM, et al. Impaired wound healing secondary to bevacizumab. Int Wound J. 2019;16:1009–12.
Kiuru M, Schwartz M, Magro C. Cutaneous thrombogenic vasculopathy associated with bevacizumab therapy. Dermatol Online J. 2014. https://doi.org/10.5070/D3206022869.
Harigai M, Nagasaka K, Amano K, et al. 2017 clinical practice guidelines of the Japan research committee of the ministry of health, labour, and welfare for intractable vasculitis for the management of ANCA-associated vasculitis. Mod Rheumatol. 2019;29:20–30.
Collins L, Seraj S. Diagnosis and treatment of venous ulcers. Am Fam Physician. 2010;81:989–96.
Burian EA, Sabah L, Karlsmark T, et al. Cytokines and venous leg ulcer healing-a systematic review. Int J Mol Sci. 2022;23:6526.
Etufugh CN, Phillips TJ. Venous ulcers. Clin Dermatol. 2007;25:121–30.
Ogawa C, Morita M, Omura A, et al. Hand-foot syndrome and post-progression treatment are the good predictors of better survival in advanced hepatocellular carcinoma treated with sorafenib: a multicenter study. Oncology. 2017;93(Suppl 1):113–9.
Wang P, Tan G, Zhu M, et al. Hand-foot skin reaction is a beneficial indicator of sorafenib therapy for patients with hepatocellular carcinoma: a systemic review and meta-analysis. Expert Rev Gastroenterol Hepatol. 2018;12:1–8.
Howell J, Pinato DJ, Ramaswami R, et al. On-target sorafenib toxicity predicts improved survival in hepatocellular carcinoma: a multi-centre, prospective study. Aliment Pharmacol Ther. 2017;45:1146–55.
Miller KK, Gorcey L, McLellan BN. Chemotherapy-induced hand-foot syndrome and nail changes: a review of clinical presentation, etiology, pathogenesis, and management. J Am Acad Dermatol. 2014;71:787–94.
Acknowledgements
This work was supported by Research Funds to Promote Hospital Functions Provided by the Japan Organization of Occupational Health and Safety.
Author information
Authors and Affiliations
Contributions
YH: conceptualization, resources, writing original draft, review, and editing, RK: conceptualization, resources, writing original draft, review, editing and funding acquisition. HO, TU, MK, TI: review, and editing. HN, TM: supervision, review, and editing.
Corresponding author
Ethics declarations
Conflict of interest
The authors have no financial conflicts of interest to disclose.
Human/animal rights
All procedure followed have been performed in accordance with the ethical standards laid down in the 1964 Declaration of Helsinki and its later amendments.
Informed consent
This study does not contain identifying information of the patients.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Hayashi, Y., Kaneko, R., Ogino, H. et al. A case of multiple hepatocellular carcinoma experiencing complete responses to sorafenib and atezolizumab–bevacizumab and developing severe, refractory venous congestive cutaneous ulcers on either regimen. Clin J Gastroenterol 16, 229–236 (2023). https://doi.org/10.1007/s12328-023-01756-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12328-023-01756-3