Abstract
Anal squamous cell carcinoma (ASCC) is an uncommon tumor. However, its incidence is increasing worldwide. Surgical resection of locally advanced cases requires permanent anal prosthesis. Thus, chemoradiotherapy (CRT) is preferred as the first-line treatment; however, high local recurrence rate remains an issue. Here, we describe two cases of locally advanced ASCC treated with docetaxel + cisplatin + S-1 (DCS) followed by CRT with S-1 that showed complete response. The two patients, aged 69 and 65 years, were diagnosed with ASCC (cStage IIIB) at our hospital. Due to extensive lymph node metastases, the patients were treated with triple induction chemotherapy (DCS) followed by CRT with S-1. Positron emission tomography/computed tomography performed six months after starting the treatment showed disappearance of tumors, indicating a complete response. The patients continued to receive S-1 for one year and achieved relapse-free long-term survival since the completion of treatment. Therefore, induction chemotherapy with DCS, prior to CRT with S-1 may benefit patients with locally advanced ASCC.







Similar content being viewed by others
Data availability
The data that support the findings of this study are available on request from the corresponding author, YS.
References
Julie DR, Goodman KA. Advances in the management of anal cancer. Curr Oncol Rep. 2016;18:20.
Altekruse SF, Kosary CL, Krapcho M, et al. (2010) SEER cancer statistics [review] 975–2007
Sauter M, Keilholz G, Kranzbühler H, et al. Presenting symptoms predict local staging of anal cancer: a retrospective analysis of 86 patients. BMC Gastroenterol. 2016;16:46.
Tanum G, Tveit K, Karlsen KO. Diagnosis of anal carcinoma—doctor’s finger still the best? Oncology. 1991;48:383–6.
Sameshima S, Sawada T, Nagasako K. Squamous cell carcinoma of anus and carcinoma in association with anal fistula in Japan, multi-institutional registration. J Jpn Soc Coloproctol. 2005;58:4.
Martin D, Balermpas P, Winkelmann R, et al. Anal squamous cell carcinoma—state of the art management and future perspectives. Cancer Treat Rev. 2018;65:11–21.
Nigro ND, Seydel HG, Considine B, et al. Combined preoperative radiation and chemotherapy for squamous cell carcinoma of the anal canal. Cancer. 1983;51:1826–9.
Takashima A, Shimada Y, Hamaguchi T, et al. Current therapeutic strategies for anal squamous cell carcinoma in Japan. Int J Clin Oncol. 2009;14:416–20.
Northover J, Glynne-Jones R, Sebag-Montefiore D, et al. Chemoradiation for the treatment of epidermoid anal cancer: 13-year follow-up of the first randomised UKCCCR Anal Cancer Trial (ACT I). Br J Cancer. 2010;102:1123–8.
Peiffert D, Tournier-Rangeard L, Gérard JP, et al. Induction chemotherapy and dose intensification of the radiation boost in locally advanced anal canal carcinoma: final analysis of the randomized UNICANCER Accord 03 trial. J Clin Oncol. 2012;30:1941–8.
Gunderson LL, Winter KA, Ajani JA, et al. Long-term update of US GI intergroup RTOG 98–11 phase III trial for anal carcinoma: survival, relapse, and colostomy failure with concurrent chemoradiation involving fluorouracil/mitomycin versus fluorouracil/cisplatin. J Clin Oncol. 2012;30:4344–51.
Oiwa S, Takayama T, Yoshida M, et al. Metabolic complete response after docetaxel, cisplatin and S-1 (DCS) therapy and sequential radiation therapy in a patient with anal squamous cell carcinoma: a case report. Clin Oncol Res. 2018. https://doi.org/10.31487/j.COR.2018.10.011.
Nelson RA, Levine AM, Bernstein L, et al. Changing patterns of anal canal carcinoma in the United States. J Clin Oncol. 2013;31:1569–75.
Ryan DP, Compton CC, Mayer RJ. Carcinoma of the anal canal. N Engl J Med. 2000;342:792–800.
Eng C, Ahmed S. Optimal management of squamous cell carcinoma of the anal canal: where are we now? Expert Rev Anticancer Ther. 2014;14:877–86.
Benson AB, Venook AP, Al-Hawary MM, et al. Anal carcinoma, version 2.2018, NCCN clinical practice guidelines in oncology. J Natl Compr Canc Netw. 2018;16:852–71.
Tomaszewski JM, Link E, Leong T, et al. Twenty-five-year experience with radical chemoradiation for anal cancer. Int J Radiat Oncol Biol Phys. 2012;83:552–8.
Leon O, Guren M, Hagberg O, et al. Anal carcinoma—survival and recurrence in a large cohort of patients treated according to Nordic guidelines. Radiother Oncol. 2014;113:352–8.
Ben-Josef E, Moughan J, Ajani JA, et al. Impact of overall treatment time on survival and local control in patients with anal cancer: a pooled data analysis of radiation therapy oncology group trials 87–04 and 98–11. J Clin Oncol. 2010;28:5061–6.
Nilsson PJ, Svensson C, Goldman S, et al. Epidermoid anal cancer: a review of a population- based series of 308 consecutive patients treated according to prospective protocols. Int J Radiat Oncol Biol Phys. 2005;61:92–102.
Moureau-Zabotto L, Viret F, Giovaninni M, et al. Is neoadjuvant chemotherapy prior to radio-chemotherapy beneficial in T4 anal carcinoma? J Surg Oncol. 2011;104:66–71.
Kim S, François E, André T, et al. Docetaxel, cisplatin, and fluorouracil chemotherapy for metastatic or unresectable locally recurrent anal squamous cell carcinoma (Epitopes-HPV02): a multicentre, single-arm, phase 2 study. Lancet Oncol. 2018;19:1094–106.
Sato Y, Takayama T, Sagawa T, et al. Phase II study of S-1, docetaxel and cisplatin combination chemotherapy in patients with unresectable metastatic gastric cancer. Cancer Chemother Pharmacol. 2010;66:721–8.
Hirakawa M, Sato Y, Ohnuma H, et al. A phase II study of neoadjuvant combination chemotherapy with docetaxel, cisplatin, and S-1 for locally advanced resectable gastric cancer: nucleotide excision repair (NER) as potential chemoresistance marker. Cancer Chemother Pharmacol. 2013;71:789–97.
Sato Y, Ohnuma H, Nobuoka T, et al. Conversion therapy for inoperable advanced gastric cancer patients by docetaxel, cisplatin, and S-1 (DCS) chemotherapy: a multi-institutional retrospective study. Gastric Cancer. 2017;20:517–26.
Ojima T, Nakamura M, Nakamori M, et al. Triplet chemotherapy with docetaxel, cisplatin and S-1 for unresectable advanced squamous cell carcinoma of the esophagus: phase I/II trial results. Oncotarget. 2019;10:847–55.
Bae WK, Hwang JE, Shim HJ, et al. Multicenter phase II study of weekly docetaxel, cisplatin, and S-1 (TPS) induction chemotherapy for locally advanced squamous cell cancer of the head and neck. BMC Cancer. 2013;13:102.
Acknowledgements
We would like to thank Dr. Takeshi Kuroda (Department of Surgery, Tokushima Municipal Hospital) and Dr. Seisuke Okamura (Okamura Clinic) for referring the patients.
Author information
Authors and Affiliations
Contributions
MG, YS did literature research and wrote the manuscript; KO, AK, TK, KN, KK, YM, HM, and TT were part of the management team for the patients. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
All authors declare that they have no conflicts of interest to disclose.
Human rights
All followed procedures were conducted in accordance with the ethical standards set out in the 1964 Declaration of Helsinki and its subsequent amendments.
Informed consent
Informed consent was provided by all patients for the present cases.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
12328_2022_1736_MOESM1_ESM.pdf
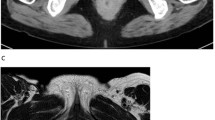
Supplementary file1 Positron emission tomography/computed tomography (PET–CT) images six months after starting treatment for patient #2 (PDF 42 KB)
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Yamasaki, M., Sato, Y., Okamoto, K. et al. Two cases of anal squamous cell carcinoma achieving complete response after docetaxel + cisplatin + S-1 (DCS) induction chemotherapy followed by chemoradiation. Clin J Gastroenterol 16, 180–186 (2023). https://doi.org/10.1007/s12328-022-01736-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12328-022-01736-z