Abstract
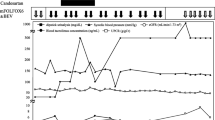
Advances in cancer chemotherapy have increased the opportunities of treating patients with cancer with renal dysfunction. Here we report the case of a 64-year-old woman with recurrent colorectal cancer who was treated with bevacizumab (BEV) combination chemotherapy. Although proteinuria caused by BEV developed early during the treatment and her renal function gradually deteriorated, BEV combination chemotherapy could be continued for 48 cycles over 2.5 years for controlling disease progression without other adverse events such as hypertension, decreased serum albumin level, or edema. After BEV discontinuation, proteinuria gradually improved and further renal function deterioration was not observed. Because the therapeutic options available for metastatic colorectal cancer are limited, balancing the risks and benefits of continuing chemotherapy is important in cases of adverse events.

Similar content being viewed by others
References
Launay-Vacher V, Oudard S, Janus N, et al. Prevalence of renal insufficiency in cancer patients and implications for anticancer drug management: the renal insufficiency and anticancer medications (IRMA) study. Cancer. 2007;110:1376–84.
Funakoshi T, Horimatsu T, Nakamura M, et al. Chemotherapy in cancer patients undergoing haemodialysis: a nationwide study in Japan. ESMO Open. 2018;3:e000301.
Kitai Y, Matsubara T, Yanagita M. Onco-nephrology: Current concepts and future perspectives. Jpn J Clin Oncol. 2015;45:617–28.
Ferrara N, Gerber HP, LeCouter J. The biology of VEGF and its receptors. Nat Med. 2003;9:669–76.
Avastin Prescribing Information. [Internet]. [cited 2018 Nov 26]. https://www.gene.com/download/pdf/avastin_prescribing.pdf
Stintzing S, Modest DP, Rossius L, et al. FOLFIRI plus cetuximab versus FOLFIRI plus bevacizumab for metastatic colorectal cancer ( FIRE-3): a post-hoc analysis of tumour dynamics in the fi nal RAS wild-type subgroup of this randomised open-label phase 3 trial. Lancet Oncol. 2016;17:1426–34.
Loupakis F, Cremolini C, Masi G, et al. Initial therapy with FOLFOXIRI and bevacizumab for metastatic colorectal cancer. N Engl J Med. 2014;371:1609–18.
Yoshino T, Arnold D, Taniguchi H, et al. Pan-Asian adapted ESMO consensus guidelines for the management of patients with metastatic colorectal cancer: a JSMO-ESMO initiative endorsed by CSCO, KACO, MOS. SSO and TOS. Ann Oncol. 2018;29:44–70.
Zhang B, Fang C, Deng D, Xia L. Research progress on common adverse events caused by targeted therapy for colorectal cancer (Review). Oncol Lett. 2018;16:27–33.
Izzedine H, Massard C, Spano JP, Goldwasser F, Khayat D, Soria JC. VEGF signalling inhibition-induced proteinuria: Mechanisms, significance and management. Eur J Cancer. 2010;46:439–48.
Kandula P, Agarwal R. Proteinuria and hypertension with tyrosine kinase inhibitors. Kidney Int. 2011;80:1271–7.
Cunningham D, Lang I, Marcuello E, et al. Bevacizumab plus capecitabine versus capecitabine alone in elderly patients with previously untreated metastatic colorectal cancer (AVEX): An open-label, randomised phase 3 trial. Lancet Oncol. 2013;14:1077–85.
Wu S, Kim C, Baer L, Zhu X. Bevacizumab increases risk for severe proteinuria in cancer patients. J Am Soc Nephrol. 2010;21:1381–9.
Chinot OL, Wick W, Mason W, et al. Bevacizumab plus radiotherapy-temozolomide for newly diagnosed glioblastoma. N Engl J Med. 2014;370:709–22.
Lafayette RA, Mccall B, Li N, et al. Incidence and relevance of proteinuria in bevacizumab-treated patients: pooled analysis from randomized controlled trials. Am J Nephrol. 2014;40:75–83.
Usui J, Glezerman IG, Salvatore SP, Chandran CB, Flombaum CD, Seshan SV. Clinicopathological spectrum of kidney diseases in cancer patients treated with vascular endothelial growth factor inhibitors: a report of 5 cases and review of literature. Hum Pathol. 2014;45:1918–27.
Acknowledgements
The authors express their sincere gratitude to all the staff members involved in this case. The authors would like to thank Enago (https://www.enago.jp) for the English language review.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Manabu Muto received a research grant from Chugai Pharmaceutical Co Ltd. outside of this research.
Human rights
All procedures followed have been performed in accordance with the ethical standards laid down in the 1964 Declaration of Helsinki and its later amendments.
Informed consent
Informed consent was obtained from all patients for being included in the study.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Kataoka, S., Nishikawa, Y., Funakoshi, T. et al. Long-term survival and renal dysfunction in a patient with recurrent colorectal cancer treated with Bevacizumab. Clin J Gastroenterol 13, 316–319 (2020). https://doi.org/10.1007/s12328-019-01060-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12328-019-01060-z