Abstract
Eosinophilic esophagitis (EoE) is a chronic and abnormal Th2 type immunological response characterized by intense eosinophilic inflammation localized within the esophagus. This leads to esophageal dysfunction and remodeling accompanied by subepithelial fibrosis. Recently, EoE has been recognized as one of the major causes of dysphagia or food impaction in adults. The prevalence of EoE has been increasing over the past several decades, particularly in Western countries. EoE should be differentiated from secondary esophageal eosinophilia (EE) in gastroesophageal reflux disease (GERD) and eosinophilic gastroenteritis, involving the entire gastrointestinal tract. EoE is an uncommon condition in Asia compared with Western countries. With the growing interest and awareness of this condition during the past decade, reports of this disease are increasingly emerging in Asian countries including Japan. Typical EoE does not respond to proton pump inhibitor (PPI) therapy according to the current Western diagnostic guidelines. However, some cases of EE exhibit symptomatic relief and histological improvement in response to PPI [i.e., PPI-responsive esophageal eosinophilia (PPI-REE)]. The understanding of the clinical manifestations and unique endoscopic images of EoE, differences and similarities between GERD, PPI-REE, and EoE will all serve as the differential diagnosis. Further knowledge of the indications and efficacy of PPI therapy and topical steroid therapy will also aid in the management of these diseases. In this article, we will review the current diagnosis and treatment of EoE in clinical practice.
Similar content being viewed by others
Epidemiology
Esophageal eosinophilia (EE) was first reported as the esophageal involvement of eosinophilic gastroenteritis (EGE) in 1977 [1] or as a partial manifestation of esophageal achalasia in 1978 [2]. Thereafter, this condition had been considered a subtype of gastroesophageal reflux disease (GERD) with atypical appearance, termed “ringed esophagus” [3]. In 1993, Attwood et al. [4] described a case series of 12 patients characterized by dysphagia as a chief complaint, young male predominance, intense eosinophilic inflammation localized in the esophagus, normal endoscopy, and no pathological gastro-esophageal reflux. Their report was the first to establish EoE as a distinct disease entity different to GERD or secondary EE (e.g., EGE, hypereosinophilic syndrome, celiac disease, Crohn’s disease, infection, drug hypersensitivity, or connective tissue disease) [5].
The majority of the epidemiological studies from Western countries have shown that the prevalence of EoE has been increasing during recent decades. The prevalence of EoE is likely influenced by an enhanced recognition of this condition and increasing endoscopy use in clinical practice [6–9]. The prevalence of EoE is much higher in Western countries compared to Asia, including Japan. In contrast, EGE is relatively common in the Asian population compared to the Western population [10–12]. A recent systematic review by Arias et al. [13] revealed that the population-based prevalence of EoE was estimated at 2.3–56.3/100,000, with a combined prevalence of 22.7/100,000 and 0.28% in North American and European populations. As expected, the prevalence varies widely by the population investigated in each study. According to several previous reports, EoE was diagnosed in 0.02–0.4% in the general population [6, 14], in 1.0–6.5% among patients with upper endoscopy for various reasons [15–17], and in 2–48% among the patients with dysphagia or a food impaction [18–22]. In addition, there is a report that EoE is more prevalent in areas with a cold or arid climate, as well as urban areas compared with warm climates or rural areas [23, 24]. Thus, there is a wide variation in the reported prevalence of EoE according to the various factors, such as the sample size, studied population, and area.
Although EoE has been reported in all generations, the prevalence between children and adults is similar [13, 25, 26]. In adults, these conditions are two- or threefold more common in men, presenting primarily in young men ranging from 20 to 40 years of age [27]. Individuals with EoE frequently have allergic conditions (e.g., bronchial asthma, atopic dermatitis, or rhinitis), with an overall prevalence of 60–70% in the US population [25, 26].
In Japan, EoE was first reported by Furuta et al. [28]. The prevalence of EE or EoE is estimated to be 0.02–6.6% (Table 1) in the recent studies reported from the east Asia including Japan, in which studied population have undergone an endoscopy for a health check-up or for gastrointestinal symptoms suggestive of EoE [29–37]. The number of the patients enrolled in Asian reports is approximately 20 at most, much lower than that in the Western reports. According to the recent meta-analysis by Kinoshita et al. [38] the reported prevalence of EoE in Asian population is approximately 17.1-6557/100,000, exhibiting a wide range possibly attributable to an inclusion bias with studies using a small sample size or a different indication for endoscopy. Allergic predisposition and male predominance are similarly observed in Japan, similar to Western countries; however, the peak age at diagnosis ranges from 40 to 60 years, which is an older age compared with that of the Western patients [11, 39]. “Asymptomatic EoE” without any troublesome esophageal symptoms is occasionally and incidentally identified in clinical practice in Japan, where the screening endoscopy is widely and easily performed [30, 32–34, 40, 41]. However, this type of EoE is not an entity according to the current diagnostic guidelines. Moreover, little is known about the prevalence, clinical significance, and natural history of asymptomatic subjects with EE.
Pathogenesis
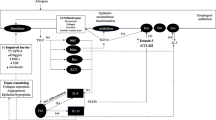
A growing body of experimental and clinical evidence supports the notion that an enhanced Th2 type-immunological reaction against causal food allergens, with or without a potential trigger by aeroallergens, is the main underlying mechanism of EoE [42, 43]. This reaction occurs primarily via non-IgE mediated hypersensitivity (delayed hypersensitivity), with a partial participation of IgE-mediated hypersensitivity (immediate hypersensitivity) [44–46]. Acute EE associated with an immediate food hypersensitivity is reportedly present, but it is an uncommon condition [47, 48]. In brief, when causal allergens are ingested and exposed to the esophageal epithelium, these subsequently permeate to the subepithelium, triggering the activation of dendritic cells through the induction of thymic stromal lymphoprotein (TSLP). TSLP is a key cytokine related to the induction and enhancement of the Th2 type immunological reaction mainly produced by epithelial cells and basophils [49]. Activated dendritic cells strongly induce the proliferation of Th2 cells, which leads to an increase in several cytokines associated with eosinophilic inflammation, (e.g., IL-5, IL13, or IL15) [42]. IL-5 differentiates and recruits intramedullary eosinophils or those in the peripheral intravascular compartment [50]. IL-13 and IL-15 induce the secretion of eotaxin-3 from epithelial cells, which is one of the strongest chemotactic factors for eosinophils [45]. IL-13 also attenuates the barrier function of the squamous epithelium by decreasing the gene expression of the epidermal differentiation complex (e.g., filaggrin or involucrin) [51]. Locally aggregated and activated eosinophils, in conjunction with mast cells, produce TGF-β1. This in conjunction with the action of fibroblasts and periostin, triggers fibrotic changes in the esophageal wall, leading to the dysfunction of the smooth muscle [42]. It has also been shown by candidate gene studies and genome-wide association investigations, that single nucleotide polymorphisms exist in the disease-related genes encoding eotaxin-3, TGF-β1, filaggrin, TSLP, and the TSLP receptor [42]. Thus, a subject who has genetic risk factors associated with eosinophilic inflammation would be susceptible to EoE once exposed to causal food and/or environmental allergens [52].
Clinical manifestations
The clinical symptoms of EoE are considerably different between children and adults [53]. In children, unspecific symptoms (e.g., heartburn, nausea, vomiting, abdominal pain, or failure to thrive) are presented in addition to dysphagia, while in adults, eating difficulties (e.g., repeated dysphagia or food impaction) are predominantly presented [54]. This difference appears to be associated with the time-dependent disease progression in which active eosinophilic inflammation is predominantly present in children and subsequent fibrostenotic changes of the esophageal wall are the main complications in adults [55, 56]. Dysfunction of the esophageal proper muscle layer is also considered to participate in symptom generation [57, 58]. However, subepithelial fibrosis or muscle dysfunction is difficult to detect by a conventional endoscopic approach with a biopsy. This may partially explain the discrepancy between the degree of clinical symptoms and the severities of endoscopic abnormalities or histological eosinophilic inflammation in EoE [59–62].
Esophageal foreign body impaction is a major GI emergency in Western countries, where EoE with food impaction is more common. In a report by Sarah et al. approximately 100 patients per year present at their university hospital with an esophageal foreign body impaction. Approximately half of these cases are caused by a food impaction. Furthermore, half of these food impaction cases are histologically diagnosed with EoE by esophageal biopsies [22]. The most commonly impacted foods in EoE are meat products, such beef or chicken [63]. Interestingly, food impaction or esophageal strictures are much more uncommon in Japan [34, 38]. Although a rare occurrence, esophageal perforation can occur with the patient’s effort to vomit the impacted foods [64].
There is a wide variation in the severity of clinical symptoms among EoE patients, ranging from no remarkable symptoms, occasional dysphagia with certain solid foods, to repeated food impaction almost daily. When dysphagia is mild and infrequent, the patient may not consult a doctor, likely considering the symptoms as part of their constitutional property. Thus, it is likely that this condition is underdiagnosed or that the diagnosis is delayed [56]. We also need to take into account that patients with EoE often have behavioral alternations, such as avoiding the foods previously responsible for dysphagia or impaction. Moreover, these patients tend to eat more slowly, drink water while eating for ease of swallowing in a conscious or an unconscious way, potentially leading to the underestimation of disease activity.
Some adult patients with EoE primarily complain of heartburn in addition to dysphagia or a food impaction. Thus, in these cases, it is difficult to discriminate between EoE and GERD based solely on the patient’s symptoms. The majority of patients with GERD can achieve symptomatic relief and endoscopic improvement by acid suppressive therapy using PPI, whereas typical EoE does not respond to PPI. Therefore, EoE has been recognized as a different diagnostic entity of refractory reflux disease. Previous studies have revealed that the prevalence of EoE is almost as high as 10% among PPI-failed reflux disease [65–67]. It is important to know that EoE is a potential cause of GERD-related symptoms unresponsive to adequate PPI therapy.
Definition
EoE has been categorized as an eosinophilic gastrointestinal disorder which consists of EoE, EGE, and eosinophilic colitis (EC). In EoE, eosinophilic inflammation is localized to the esophagus; however, in the EGE or EC, it can extend to the entire gastrointestinal tract from the esophagus to the rectum [10]. EoE has been recognized as a clinicopathological disease characterized by both esophageal symptoms and histologically proven EE. According to the updated guideline proposed in the US and Europe: (1) the presence of any esophageal symptoms (e.g., dysphasia, food impaction, heartburn, or chest pain); (2) EE with a peak of more than 15 eosinophils/high-power field (HPF); (3) unresponsiveness to profound acid suppressive therapy using PPI; and (4) the exclusion of secondary EE (e.g., EGE, hypereosinophilic syndrome, connective tissue disease, infection, drug hypersensitivity, or Crohn’s disease) are the diagnostic criteria for EoE [27]. Unresponsiveness to PPI was originally included in the early diagnostic criteria to exclude GERD as a potential cause of eosinophilic inflammation [5]. However, in practice, an overlap between EoE and GERD may be pathophysiologically possible as EoE can occur secondary to abnormal reflux by inducing esophageal dysfunction. Furthermore, GERD can trigger eosinophilic inflammation by increasing an intraepithelial permeation of causal allergens through dilated intercellular space of the injured esophageal epithelium [68]. Indeed, a recent meta-analysis revealed that 20% of patients with EoE have erosive esophagitis. Thus, EoE is not ruled out by concurrent GERD. As mentioned below, EE is responsive to PPI, namely “PPI-REE,” is recommended to be discriminated from EoE unresponsive to PPI in the current diagnostic guidelines proposed in Western countries [27, 69].
In Japan, EoE is simply defined by the presence of clinical symptoms and the histological demonstration of EE according the diagnostic criteria proposed by the EoE/EGE study group committee of the Ministry of Health, Labour, and Welfare [11] (Table 2). Unlike Western countries, the unresponsiveness to PPI is presented as an adjunct; however, it is not an essential finding, which supports the diagnosis of EoE in addition to characteristic endoscopic findings, the thickness of the esophageal wall, peripheral eosinophilia, and male predominance. A cutoff threshold of infiltrating eosinophils is determined with 15 eosinophils/HPF (400×) according to the Western criteria [70]. It has been reported that 10% of Japanese patients with EGE have eosinophilic inflammation involving the esophagus [71]. Under such conditions, EE associated with EGE is classified as EGE, but not as EoE. When an adult patient with suspected EoE has abdominal symptoms (e.g., nausea, vomiting, and diarrhea or abnormal endoscopic gastrointestinal tract findings), additional biopsies from the stomach or the intestine should be obtained for a further histological assessment and diagnose EGE, if present [69].
Diagnosis
Blood and allergy testing
Peripheral eosinophils and the total serum IgE levels are elevated in 10–50% and 60–70% of adult EoE patients, respectively, in Western countries [5]. In adult Japanese patients with EoE, an peripheral eosinophilia was found in only 10–30% of patients with EoE [11, 31], while the total level of IgE was elevated in 50–80% [31, 72]. There are some reports showing that the measurement of peripheral eosinophils may be useful for monitoring the activity of EoE, at least in a subgroup of patients [73, 74]. Because of the high prevalence of concurrent atopic conditions or its various activities, it is difficult to determine a causal relationship between the level of IgE and EoE activity.
To date, aeroallergen- and food-specific IgE can be simultaneously and easily measured by a chemiluminescence enzyme immunoassay, providing relevant information regarding an immediate-type allergic diathesis to multiple allergens. It has been reported that aeroallergen-specific IgE and food-specific IgE are present in 60–80% and 40–80% of adult EoE patients, respectively [72, 75]. To evaluate directly the causal allergens, two types of skin tests have been developed: (1) the skin patch test (SPT); and (2) the atopy patch test (APT). The SPT assesses the status of the immediate-type allergic response as serum-specific IgE, while the APT judges the status of the delayed-type allergic response. Based on these skin tests, several foods, including peanut, egg, soybean, cow’s milk, and tree nuts, have been identified as the most common food allergens in EoE [76]. Recently, empiric elimination diet therapy that antecedently avoiding these major allergic foods has been developed and reported to be useful as a nonpharmacological therapeutic option as described below [77–79].
Endoscopic findings
Several endoscopic findings, including linear furrows, concentric rings, white exudates, decreased vasculature in the esophageal mucosa, esophageal strictures, and the esophagus of narrow caliber, have been reported to be the characteristic findings of EoE, although neither of these is specific [80]. Endoscopy and subsequent biopsies are the most critical assessments for the diagnosis of EoE, in which EE can be histologically proven. Thus, endoscopists need to be familiar with the above representative image.
Linear furrows are longitudinally observed in the esophagus, being relatively frequent and pathognomonic compared with other endoscopic findings (Fig. 1) [33, 80]. Concentric rings are horizontally observed along the short axis of the esophagus, which is referred to as “trachealization” or “ringed esophagus” in severe cases (Fig. 2). However, these rings should be evaluated with caution since subtle or transient rings are occasionally present in patients with GERD or even in normal subjects with a potent gag reflex during the endoscopic examination. This feature has also been previously described as feline esophagus [81, 82]. White exudates histologically correspond to eosinophilic microabscesses with the aggregation of a couple of eosinophils [83], which grossly resemble esophageal candidiasis (Fig. 3) [84, 85]. The esophageal mucosa appears to be thick and whitish, owing to the marked inflammation and edema, resulting in decreased or missed vascularity, often observed in GERD. Multiple polypoid lesions resembling esophageal papilloma may be present in some adolescent and adult patients (Fig. 4) [87, 88]. Subepithelial fibrosis in the esophageal wall progresses with persisting long-term eosinophilic inflammation, resulting in an esophagus of narrow caliber or with an esophageal stricture [55, 56]. In some cases, a traumatic tear occurs with the passage of the endoscope, namely crepe-paper esophagus, indicative of mucosal fragility (Fig. 5) [86]. This can occur in both the lower esophagus, as well as in the middle or upper esophagus, in contrast to GERD [89–91]. In some case, most of whom have no symptom, these endoscopic abnormalities is localized in a narrow area just above the esophagogastric junction (Fig. 6) [37, 40].
Linear furrows run along the longitudinal axis of the esophagus. A White light image. B Narrow-band imaging. C Indigo carmine-sprayed image. D Linear erosion with reflux esophagitis (white arrows) is distinguishable from linear furrows with EoE (while arrow heads). E Double line or fissure-like furrows are easily recognized when in contact with blood after esophageal biopsies are obtained. F Cobble-stone like appearance is present in the linear furrows in severe cases
Endoscopic abnormalities, such as linear furrows, concentric rings, or decreased vascularity, are localized in a small area above the esophagogastric junction (A, B). Marked eosinophilic infiltration is histologically proven in these localized cases (figure not shown), as well as in typical histological findings of EoE as shown in Fig. 7
According to a meta-analysis conducted by Kim et al. consisting primarily of retrospective studies involving adult cohorts, the overall pooled prevalence of endoscopic findings in patients with EoE was 44% rings, 21% strictures, 9% narrow caliber esophagus, 48% linear furrows, 27% white exudates, and 41% decreased vascularity, with a wide variation in the prevalence of those endoscopic findings between each report. This variation can be ascribed to a lack of an established diagnostic system via endoscopy in EoE, as indicated by the unsatisfactory inter-observer agreement for the endoscopic findings [92]. On the other hand, when only the prospective studies were examined in the meta-analysis, 93% of patients were found to have at least one endoscopic abnormality.
A recently proposed classification and grading system using the major (i.e., edema, rings, exudates, furrows, and strictures) and the minor (i.e., feline, narrow caliber, and crepe paper esophagus) endoscopic features was reported to have good inter- and intra-observer agreement, except for edema and feline esophagus, irrespective of whether the assessment was performed by expert or non-expert endoscopists [86, 93]. This diagnostic system, termed the EREFS system (E, edema; R, rings; E, exudates; F, furrows; and S, strictures) is expected to contribute to the objective and comprehensive assessment of disease severity and response to therapeutic intervention [94]. The existence or its severity of these individual endoscopic signs do not appear to correlate well with the degree of infiltrating eosinophils [95, 96]; however, it was shown in one retrospective study, that there was a weak to moderate correlation between the combined use of these signs with the histological disease activity [95]. This suggests that the coexistence of multiple endoscopic abnormalities increase the possibility of a histologically definitive diagnosis of EoE, but unfortunately its sensitivity and negative predictive value for histological disease activity was low (20–30%) in that study. On the other hand, in a recent prospective study by Dellon et al. [96] a significant correlation was found between a decreased total and individual EREFS score (with the exception of rings) and histological response following treatment. These inconsistent results may reflect that clinical studies exploring the correlation between the endoscopic and histologic status are potentially impacted by various factors, such as the study design, inter-observer variation in endoscopic evaluation, biopsy protocol, and the method of treatment. It may be difficult to estimate the histological disease activity from the endoscopic signs with a high accuracy for all patients and disease status.
There is a previous report demonstrating that approximately 40% of patients with EoE have been misdiagnosed with Schatzki’s rings, an esophageal stricture/web, or reflux disease [97]. A recent meta-analysis showed that the endoscopic examination was normal in 20 and 7% of patients from retrospective and prospective analyses, respectively [80]. Prasad et al. [19] reported that EoE was diagnosed in 10% of patients with dysphagia and a normal-appearing esophageal mucosa. Therefore, endoscopists should obtain esophageal biopsies even if there are no remarkable endoscopic abnormalities, particularly for patients with unexplained dysphasia or food impaction.
In contrast, it has been reported that the presence of typical endoscopic abnormalities suggestive of EoE added to the presence of clinical symptoms yield a four- to fivefold increase in the possibility of being diagnosed with EoE [19, 21]. Shimura et al. [33] showed that in symptomatic Japanese patients suspected of having EoE, seven out of 30 patients (23.3%) with EoE-suggestive endoscopic abnormalities were diagnosed with EoE, whereas EoE was found in only one out of 289 patients (0.34%) without such endoscopic findings. Lutein esophageal biopsies should not always be recommended for all of patients with dysphagia in the absence of endoscopic abnormalities, especially in populations with a lower prevalence of EoE, such as Asian populations.
Histological findings
The histological demonstration of EE is essential for the diagnosis of EoE. Since there are virtually no eosinophils in the normal esophageal epithelium [98, 99], even a few infiltrating eosinophils are considered to be pathogenic. As mentioned above, pathological eosinophilic inflammation can be secondarily induced by various causes, such as GERD, EGE, hyper-eosinophilic syndrome, Crohn’s disease, celiac disease, connective tissue disease, achalasia, infection, drugs, or a graft-versus-host reaction [69]. Of these, GERD is considered to be the most common cause of secondary EE in clinical practice. It is conceivable that up to 10 eosinophils can emerge in the esophagus by GERD based on previous studies [4, 100, 101]. Several cutoff values of eosinophil counts (i.e., 15, 20, or 24 eosinophils/HPF) have been used as the histological definition for discriminating EoE from EE associated with GERD [102]. Recently, a peak of 15 eosinophils/HPF or more in at least one biopsied site has been defined as the diagnostic minimum threshold in the majority of clinical studies according to the updated global diagnostic consensus and recommendations for EoE [27]. A representative histological image is presented in Fig. 7. The superficial distribution of infiltrating eosinophils in the esophageal epithelium, degranulation of eosinophils, aggregation of eosinophils (eosinophilic microabscess), and lamina propria fibrosis are comparably pathognomonic of EoE [83]. In addition, basal cell hyperplasia, papilla elongation, and dilated intercellular space are commonly observed in both EoE and GERD [83].
A representative histological image observed in EoE. A Marked epithelial thickness with basal cell hyperplasia, papilla elongation, intraepithelial infiltration of eosinophils, and subepithelial fibrosis. B Many eosinophilic infiltrates (>15 eosinophils/HPF) in the epithelium with the degranulation of eosinophils, dilated intracellular space, and edema
Eosinophils infiltrating the esophageal epithelium distribute more heterogeneously in EoE [103, 104]. A previous report showed that a diagnostic sensitivity of 40–50% by one biopsy increased to almost 100% with five or more biopsies [97]. Thus, two to four esophageal biopsies should be recommended both from the proximal and the distal esophagus [69]. As mentioned above, the importance of random biopsies has been highlighted, irrespective of endoscopic findings since some EoE patients present with apparently normal mucosa via endoscopy [80]. However, the degree of EE is more intense in the areas containing white exudates [34, 105], in linear furrows [32, 106], or in the lower esophagus compared with the proximal region [34, 104], suggesting that the histological detection of EE may also be influenced by the site of the biopsies in addition to the number of biopsy samples obtained. When the peak of infiltrating eosinophils is <14 eosinophils/HPF in patients suspected of EoE, re-biopsies can aid in the definitive diagnosis of EoE [107].
Treatment
The therapeutic approach consists of the “3D” concept: diet, drugs, and dilation [108]. The patient is treated based on the severity of their symptoms or endoscopic findings, such as esophageal narrowing or stricture. Histological improvement is usually used as the primary outcome in clinical trial rather than symptomatic improvement due to the difficulty in evaluating the symptoms objectively and uniformly. It is common for these patients to modify the patient’s dietary or eating behavior to avoid dysphagia or an impaction [109].
To date, some issues regarding the treatment and management of EoE remain unresolved. First, the goal of treatment remains to be determined. Should the aim for treatment be a symptomatic remission, histological remission, or both? Second, a discrepancy exists between symptomatic and histologic remission. Some patients do not present symptom relief even after endoscopic and histological improvement has been achieved [59, 110, 111]. This discrepancy is likely attributed to the limited response of the sub-epithelial fibrosis and remodeling to the currently available medication. Third, EE easily recurs with the discontinuation of treatment, and appropriate maintenance therapy has yet to be established.
Diet
Three different types of the diet therapy, including the elemental diet, allergy-testing based elimination diet, and empiric elimination diet, have been attempted primarily in infants and more recently, in adults [112]. Although the elemental diet using an amino acid-based formula is highly effective to induce symptomatic and histological remission, especially in infants, this approach is extremely costly and poorly tolerable as it requires a feeding tube or has unpleasant flavor, and it is unsustainable for long-term use. To resolve these drawbacks, allergy-testing based elimination diet therapy has been used. This is based on the allergic status measured by skin prick test or an atopy patch test [5]. Some studies have found a relatively high efficacy for this targeted elimination therapy. However, the ability to predict the causal allergens is relatively low using the currently available allergic tests. Gonsalves et al. [78] reported that the skin prick test predicted only 13% of foods associated with EoE in adult patients. Subsequently, the empiric elimination diet, in which the most common allergens are antecedently excluded, was devised as a more practical and simple method. A recent systematic review and meta-analysis revealed that the six-food elimination diet (i.e., wheat, milk, eggs, nuts, soy, and seafood) and the four-food group elimination diet (i.e., dairy, eggs, legumes, and wheat) achieve histological remission in 70% and 50% of patients, respectively, without differences between children and adults [112]. Thus, diet therapy may be a useful alternative therapeutic option, allowing the reduction or discontinuation of medication, especially in patients who may require long-term use of steroid therapy [113].
Drug: PPI therapy
Ngo et al. [114] described a case series of three patients whose symptoms and EE had shown nearly a complete remission with PPI. This report raised the possibility that eosinophilic inflammation can occur as a consequence of reflux disease, and suggested the necessity of the “PPI testing” before the final diagnosis of EoE. Subsequently reported retrospective and prospective studies have demonstrated that about 30–70% of adult EoE patients exhibited both symptomatic and histologic improvement of EE by PPI therapy [115]. A high dose of PPI is conventionally used twice daily or once daily for 8 weeks [116]. In the consensus recommendation for EoE in 2011, it was proposed that those conditions should be discriminated from EoE as a new potential disease entity termed “PPI-REE.” Although the underlying mechanism of PPI-REE remains unclear, two theories have been mainly proposed: (1) PPI blocks the permeation of the causal allergens from the esophageal luminal surface to the subepithelium by curing the acidic damage (e.g., dilated intracellular space or erosions in the esophageal epithelium); [68, 117, 118] and (2) PPI potentially reduces eosinophilic inflammation by suppressing Th2-associated cytokine or gene expression as topical steroids, independently of the gastric acid inhibitory effect [119, 120]. Thus, symptomatic and histological resolution of EE by PPI does not necessarily indicate the existence of GERD as a potential cause of EE [121].
A latest systematic review and meta-analysis showed that PPI therapy can achieve an overall clinical response in 60% and histological response in 50% of patients with EE, although there is poor-quality evidence, heterogeneity, and publication bias in the respective study [122]. Consequently, PPI is a reasonable first-line therapeutic agent for symptomatic EE due to its feasibility, tolerability, and paucity of side effects compared with diet or steroid therapy [116]. No significant difference has been shown regarding the treatment efficacy between children and adults, between the dose and number of PPI administered, or between the absence and the presence of GERD [122]. Although evidence of the pathogenesis of PPI-REE and EoE is accumulating, the two entities cannot be clearly differentiated solely based on patient characteristics, symptoms, endoscopic findings, histological findings with immunostaining, or molecular findings [116, 123, 124]. Thus, some experts have proposed that the responsiveness to PPI should not be included in the diagnostic criteria for EoE, and that use of the term PPI-REE should be avoided [116]. High doses of PPIs (e.g., omeprazole 40 mg twice daily) are initially used in PPI therapy; however, 70–80% of patients may maintain histological remission with lower doses of PPI (e.g., omeprazole 20 mg twice or once daily) [125, 126]. In Japan, at least 15–50% of patients achieve clinicopathological remission with the standard PPI dose used for the treatment of GERD (e.g., omeprazole 20 mg once a day, rabeprazole 10 mg once a day) [11, 29–31]. In a pediatric case series, it was reported that PPI-REE is a potentially transient phenomenon [127]. Recently, Molina-Infante et al. [125] reported that 27% of patients with PPI-REE histologically relapsed on maintenance PPI therapy and the CYP2c19 polymorphism is significantly associated with the loss of a response to the therapy, in which a relapse of EE occurred in 36% of rapid metabolizers, in contrast to only 6% of intermediate or poor metabolizers. Little is known about the long-term prognosis of PPI-REE, and such patients should be monitored after clinicopathologic remission with PPI is achieved.
Drug: steroid therapy
When symptomatic and histological remission are not achieved by PPI therapy, steroid therapy should be considered [69]. Remarkably, unlike EGE, topical therapy by swallowing inhaled corticosteroids used to treat asthma is primarily applied for EoE because of its equivalent efficacy and fewer adverse events compared with systemic corticosteroid therapy. In principle, the systemic administration of steroids is considered for patients unresponsive to topical therapy, or in patients with severe conditions whose symptoms or eosinophilic inflammation must be eliminated as early as possible. As a nebulizer, fluticasone propionate has been used primarily as an early regimen; however, recently budesonide, a viscose type, was preferably selected due to a more reliable and uniform delivery to the entire esophageal mucosa in both children and adults [128, 129]. A recommended dose of topical steroids is 440–880 μg fluticasone twice daily and 1–2 mg budesonide twice daily for 8 week as an initial duration in adults [69]. The patient is instructed to swallow the nebulized agents into the esophagus by holding his or her breath to avoid inhaling the drug into the trachea. It is preferable that viscous budesonide is used as a mixed suspension with syrup for children because of its unpleasant flavor. However, for the majority of adults, it can be taken without a sweetener. After swallowing, the patient should rinse their mouth to prevent oral candidiasis and stop eating and drinking for 30 min to 1 h to avoid washing the drug from the esophagus into the stomach.
Previous studies have demonstrated that topical steroid therapy can lead to histological remission in 15–94% and symptomatic remission in 30–97% of patients compared with the placebo, PPI, and systemic corticosteroid therapy, with an extremely wide variation in the remission rate [130]. This variation could be attributed to multiple divergent factors in each study, including the comparative agents, dosage form (nebulized or viscous), dose of the drug, patient age, sample size, treatment duration, definition of histological or symptomatic remission, and PPI trial prior to topical steroid treatment (exclusion of PPI responders) [131]. According to two recent reviews and meta-analyses, topical steroid therapy resulted in a significantly higher histological remission compared with the placebo (odds ratio 20.8–33.8), whereas the achievement of symptomatic remission induced by topical steroid therapy was only modest compared with the placebo (odds ratio 2.7–3.1) [132, 133]. As mentioned above, this discrepancy between symptomatic and histologic remission may potentially be attributed to the limited efficacy of steroid therapy on fibrostenotic changes in the subepithelium [134, 135], and difficulties in the objective assessment of clinical symptoms owing to the lack of validated symptom questionnaires [109]. Notably, in the systematic review and meta-analysis conducted by Chuang et al. [136], the significant efficacy of topical steroid therapy was observed only in patients with prior PPI therapy (PPI non-responders); this indicates the usefulness of the PPI trial prior to steroid therapy. Maintenance therapy by topical steroids is effective for controlling the clinical symptoms or eosinophilic inflammation in some patients [134, 137]; however, its withdrawal leads to relapse at a high rate [73, 138, 139].
Adverse effects appear to be less frequently associated with topical therapy compared with systemic steroid therapy. Mild (asymptomatic) oral candidiasis can occur in up to 10% of patients [136]. A recent report showed that 10% of children treated with topical steroids for more than 6 months exhibited adrenal insufficiency, measured by an adrenocorticotropic hormone stimulation test [140]. Although such cases have not been reported in adults, close attention should be paid for long-term users of topical steroids. Straumann et al. [134] have reported the efficacy of maintenance therapy using low-dose budesonide in adult EoE patients. Dose reductions should be considered as much as possible while aiming to maintain clinical remission.
Dilation
Dilation therapy is recommended for symptomatic patients who have esophageal strictures or narrowing despite medical therapy [141]. Patients requiring dilation are primarily adults since esophageal remodeling (e.g., esophageal stricture or narrowing) develops progressively during long-term and persistent eosinophilic inflammation [55, 56]. Three types of procedures have primarily been employed, (1) the simple bougie; (2) the wire-guided bougie; and (3) through-the-scope (TTS) balloon dilation [142]. In a recent retrospective study conducted by Runge et al. [143], 164 of 509 EoE patients were dilated a total of 486 times during a 12-year period at their hospital. The bougie and TTS dilation were used in approximately 20 and 80% of the cases, respectively. The TTS methods exhibited the potential to extend the esophageal lumen further than the bougie method, while no significant difference was reported regarding complications (e.g., pain, bleeding, and perforation). It is important for the endoscopists to gently and gradually dilate, since chest pain or mucosal tears can often occur secondary to esophageal mucosal fragility. The most critical complication is esophageal perforation, which can occur in up to 0.1% of cases according to some recent systematic reviews [144, 145]. Notably, this is a considerably lower rate compared with previous years. In patients with EoE, a younger age, multiple dilations, upper esophageal strictures, and the inability to pass through the strictures with the endoscope are risk factors for dilation-related adverse events [146]. The majority of patients demonstrate symptomatic improvement following dilation; however, its durability appears to be unsatisfactory. A recent large cohort study revealed that more than half of the patients with dilation underwent repeated procedures, especially within the first year [143]. The efficacy of endoscopic dilatation is not different in the presence or absence of concomitant antieosinophilic medication [147].
Conclusion
In this review article, we briefly described the epidemiology, pathogenesis, clinical manifestations, diagnostic definition, blood and allergy tests, endoscopic and histological findings, dietary and pharmacological therapy with PPI, as well as topical steroids and dilation therapy used in EoE. In Japan, this disease is presently infrequent; however, gastroenterologists and endoscopists should be aware of EoE as a major cause of dysphagia, food impaction, and esophageal strictures, in addition to GERD and esophageal cancer.
Abbreviations
- EoE:
-
Eosinophilic esophagitis
- EE:
-
Esophageal eosinophilia
- GERD:
-
Gastroesophageal reflux disease
- EGE:
-
Eosinophilic gastroenteritis
- EC:
-
Eosinophilic colitis
- PPI:
-
Proton pump inhibitor
- PPI-REE:
-
Proton pump inhibitor-responsive esophageal eosinophilia
- SPT:
-
Skin patch test
- APT:
-
Atopy patch test
- TSLP:
-
Thymic stromal lymphoprotein
- IL:
-
Interleukin
- TGF:
-
Transforming growth factor
- HPF:
-
High-power field
- CYP:
-
Cytochrome P450
- TTS:
-
Through-the-scope
- EGD:
-
Esophagogastroduodenoscopy
References
Dobbins JW, Sheahan DG, Behar J. Eosinophilic gastroenteritis with esophageal involvement. Gastroenterology. 1977;72(6):1312–6.
Landres RT, Kuster GC, Strum WB. Eosinophilic esophagitis in a patient with vigorous achalasia. Gastroenterology. 1978;74(6):1298–301.
Morrow JB, Vargo JJ, Goldblum JR, Richter JE. The ringed esophagus: histological features of GERD. Am J Gastroenterol. 2001;96(4):984–9.
Attwood SEA, Smyrk TC, Demeester TR, Jones JB. Esophageal eosinophilia with dysphagia—a distinct clinicopathologic syndrome. Dig Dis Sci. 1993;38(1):109–16.
Furuta GT, Liacouras CA, Collins MH, et al. Eosinophilic esophagitis in children and adults: a systematic review and consensus recommendations for diagnosis and treatment. Gastroenterology. 2007;133(4):1342–63.
Straumann A, Simon HU. Eosinophilic esophagitis: escalating epidemiology? J Allergy Clin Immunol. 2005;115(2):418–9.
Whitney-Miller CL, Katzka D, Furth EE. Eosinophilic esophagitis: a retrospective review of esophageal biopsy specimens from 1992 to 2004 at an adult academic medical center. Am J Clin Pathol. 2009;131(6):788–92.
Prasad GA, Alexander JA, Schleck CD, et al. Epidemiology of eosinophilic esophagitis over three decades in Olmsted County, Minnesota. Clin Gastroenterol Hepatol. 2009;7(10):1055–61.
Van Rhijn BD, Verheij J, Smout AJPM, Bredenoord AJ. Rapidly increasing incidence of eosinophilic esophagitis in a large cohort. Neurogastroenterol Motil. 2013;25(1):47–53.
Yan BM, Shaffer EA. Primary eosinophilic disorders of the gastrointestinal tract. Gut. 2009;58(5):721–32.
Kinoshita Y, Furuta K, Ishimaura N, et al. Clinical characteristics of Japanese patients with eosinophilic esophagitis and eosinophilic gastroenteritis. J Gastroenterol. 2013;48(3):333–9.
Ito J, Fujiwara T, Kojima R, Nomura I. Racial differences in eosinophilic gastrointestinal disorders among Caucasian and Asian. Allergol Int. 2015;64(3):253–9.
Arias A, Pérez-Martínez I, Tenías JM, Lucendo AJ. Systematic review with meta-analysis: the incidence and prevalence of eosinophilic oesophagitis in children and adults in population-based studies. Aliment Pharmacol Ther. 2016;43(1):3–15.
Ronkainen J, Talley NJ, Aro P, et al. Prevalence of oesophageal eosinophils and eosinophilic oesophagitis in adults: the population-based Kalixanda study. Gut. 2007;56(5):615–20.
Almansa C, Krishna M, Buchner AM, et al. Seasonal distribution in newly diagnosed cases of eosinophilic esophagitis in adults. Am J Gastroenterol. 2009;104(4):828–33.
Veerappan GR, Perry JL, Duncan TJ, et al. Prevalence of eosinophilic esophagitis in an adult population undergoing upper endoscopy: a prospective study. Clin Gastroenterol Hepatol. 2009;7(4):420–426.e2.
Sealock RJ, Kramer JR, Verstovsek G, et al. The prevalence of oesophageal eosinophilia and eosinophilic oesophagitis: a prospective study in unselected patients presenting to endoscopy. Aliment Pharmacol Ther. 2013;37(8):825–32.
Kerlin P, Jones D, Remedios MCC. Prevalence of eosinophilic esophagitis in adults with food bolus obstruction of the esophagus. J Clin Gastroenterol. 2007;41(4):356–61.
Prasad GA, Talley NJ, Romero Y, et al. Prevalence and predictive factors of eosinophilic esophagitis in patients presenting with dysphagia: a prospective study. Am J Gastroenterol. 2007;102(12):2627–32.
Ramakrishnan RCH. Eosinophilic oesophagitis in adults. Histopathology. 2008;52(7):897–900. doi:10.1111/j.1365-2982.2009.01307.x.
Mackenzie SH, Go M, Chadwick B, et al. Eosinophilic oesophagitis in patients presenting with dysphagia—a prospective analysis. Aliment Pharmacol Ther. 2008;28(9):1140–6.
Sperry SLW, Crockett SD, Miller CB, Shaheen NJ, Dellon ES. Esophageal foreign-body impactions: epidemiology, time trends, and the impact of the increasing prevalence of eosinophilic esophagitis. Gastrointest Endosc. 2011;74(5):985–91.
Hurrell JM, Genta RM, Dellon ES. Prevalence of esophageal eosinophilia varies by climate zone in the United States. Am J Gastroenterol. 2012;107(5):698–706.
Spergel JM, Book WM, Mays E, et al. Variation in prevalence, diagnostic criteria, and initial management options for eosinophilic gastrointestinal diseases in the United States. J Pediatr Gastroenterol Nutr. 2011;52(3):300–6.
Dellon ES, Jensen ET, Martin CF, Shaheen NJ, Kappelman MD. The prevalence of eosinophilic esophagitis in the United States. Clin Gastroenterol Hepatol. 2014;12(4):589–96.
Mansoor E, Cooper GS. The 2010–2015 prevalence of eosinophilic esophagitis in the USA: a population-based study. Dig Dis Sci. 2016;61(10):2928–34.
Liacouras CA, Furuta GT, Hirano I, et al. Eosinophilic esophagitis: updated consensus recommendations for children and adults. J Allergy Clin Immunol. 2011;128(1):3–20.
Furuta K, Adachi K, Kowari K, et al. A Japanese case of eosinophilic esophagitis. J Gastroenterol. 2006;41(7):706–10.
Fujishiro H, Amano Y, Kushiyama Y, Ishihara S, Kinoshita Y. Eosinophilic esophagitis investigated by upper gastrointestinal endoscopy in Japanese patients. J Gastroenterol. 2011;46(9):1142–4.
Fujiwara Y, Sugawa T, Tanaka F, et al. A multicenter study on the prevalence of eosinophilic esophagitis and PPI-responsive esophageal eosinophilic infiltration. Intern Med. 2012;51(23):3235–9.
Tomomatsu Y, Yoshino J, Inui K, et al. Clinical features of eosinophilic esophagitis: ten Japanese cases. Dig Endosc. 2013;25(2):117–24.
Hori K, Watari J, Fukui H, et al. Do endoscopic features suggesting eosinophilic esophagitis represent histological eosinophilia? Dig Endosc. 2014;26(2):156–63.
Shimura S, Ishimura N, Tanimura T, et al. Reliability of symptoms and endoscopic findings for diagnosis of esophageal eosinophilia in a Japanese population. Digestion. 2014;90(1):49–57.
Adachi K, Mishiro T, Tanaka S, Kinoshita Y. Suitable biopsy site for detection of esophageal eosinophilia in eosinophilic esophagitis suspected cases. Dig Endosc. 2016;28(2):139–44.
Shi YN, Sun SJ, Xiong LS, Cao QH, Cui Y, Chen MH. Prevalence, clinical manifestations and endoscopic features of eosinophilic esophagitis: a pathological review in China. J Dig Dis. 2012;13(6):304–9.
Joo MK, Park JJ, Kim SH, et al. Prevalence and endoscopic features of eosinophilic esophagitis in patients with esophageal or upper gastrointestinal symptoms. J Dig Dis. 2012;13(6):296–303.
Ma X, Xu Q, Zheng Y, et al. Prevalence of esophageal eosinophilia and eosinophilic esophagitis in adults: a population-based endoscopic study in Shanghai, China. Dig Dis Sci. 2015;60(6):1716–23.
Kinoshita Y. Systematic review: eosinophilic esophagitis in Asian countries. World J Gastroenterol July World J Gastroenterol. 2015;21(2127):8433–40.
Ishimura N, Shimura S, Jiao D, et al. Clinical features of eosinophilic esophagitis: differences between Asian and Western populations. J Gastroenterol Hepatol. 2015;30(S1):71–7.
Abe Y, Iijima K, Ohara S, et al. Localized esophageal eosinophilia: Is it an early manifestation of eosinophilic esophagitis or a subtype of gastroesophageal reflux disease? Dig Endosc. 2014;26(3):337–43.
Abe Y, Iijima K, Ohara S, et al. A Japanese case series of 12 patients with esophageal eosinophilia. J Gastroenterol. 2011;46(1):25–30.
Sherrill JD, Rothenberg ME. Genetic dissection of eosinophilic esophagitis provides insight into disease pathogenesis and treatment strategies. J Allergy Clin Immunol. 2011;128(1):23–32.
Holvoet S, Blanchard C. Genetic and molecular mechanisms leading to eosinophilic esophagitis. Rev Esp Enferm Dig. 2014;106(4):276–80.
Rothenberg ME. Eosinophilic gastrointestinal disorders (EGID). J Allergy Clin Immunol. 2004;113(1):11–29.
Blanchard C, Wang N, Stringer KF, Mishra A, Fulkerson PC, Abonia JP, Jameson SC, Kirby C, Konikoff MR, Collins MH, Cohen MB, Akers R, Hogan SP, Assa’ad AH, Putnam PE, Aronow BJ, Rothenberg M. Eotaxin-3 and a uniquely conserved gene-expression profile in eosinophilic esophagitis. J Clin Invest. 2006;116(2):536–47.
Simon D, Cianferoni A, Spergel JM, et al. Eosinophilic esophagitis is characterized by a non-IgE-mediated food hypersensitivity. Allergy Eur J Allergy Clin Immunol. 2016;71(5):611–20.
Martín-Muñoz MF, Lucendo AJ, Navarro M, et al. Food allergies and eosinophilic esophagitis—two case studies. Digestion. 2006;74(1):49–54.
Kim NI, Jo Y, Ahn SB, et al. A case of eosinophilic esophagitis with food hypersensitivity. J Neurogastroenterol Motil. 2010;16(3):315–8.
Comeau MR, Ziegler SF. The influence of TSLP on the allergic response. Mucosal Immunol. 2010;3(2):138–47.
Rothenberg ME. Gastrointestinal eosinophils. Allergy. 2001;56(Suppl 6):21–2.
Blanchard C, Stucke EM, Burwinkel K, et al. Coordinate interaction between IL-13 and epithelial differentiation cluster genes in eosinophilic esophagitis. J Immunol. 2010;184(7):4033–41.
Philpott H, Nandurkar S, Royce SG, Thien F, Gibson PR. Risk factors for eosinophilic esophagitis. Clin Exp Allergy. 2014;44(8):1012–9.
Straumann A, Aceves SS, Blanchard C, et al. Pediatric and adult eosinophilic esophagitis: similarities and differences. Allergy Eur J Allergy Clin Immunol. 2012;67(4):477–90.
Dellon ES, Gibbs WB, Fritchie KJ, et al. Clinical, endoscopic, and histologic findings distinguish eosinophilic esophagitis from gastroesophageal reflux disease. Clin Gastroenterol Hepatol. 2009;7(12):1305–13.
Dellon ES, Kim HP, Sperry SLW, Rybnicek DA, Woosley JT, Shaheen NJ. A phenotypic analysis shows that eosinophilic esophagitis is a progressive fibrostenotic disease. Gastrointest Endosc. 2014;79(4):577–585.e4.
Schoepfer AM, Safroneeva E, Bussmann C, et al. Delay in diagnosis of eosinophilic esophagitis increases risk for stricture formation in a time-dependent manner. Gastroenterology. 2013;145(6):1230–6.
Korsapati H, Babaei A, Bhargava V, Dohil R, Quin A, Mittal RK. Dysfunction of the longitudinal muscles of the oesophagus in eosinophilic oesophagitis. Gut. 2009;58(8):1056–62.
Roman S, Hirano I, Kwiatek MA, Gonsalves N, Chen J, Kahrilas PJ, Pandolfino JE. Manometric features of eosinophilic esophagitis in esophageal pressure topograpy. Neurogastroenterol Motil. 2011;23(3):208-e111.
Francis DL, Foxx-Orenstein A, Arora AS, et al. Results of ambulatory pH monitoring do not reliably predict response to therapy in patients with eosinophilic oesophagitis. Aliment Pharmacol Ther. 2012;35(2):300–7.
Kwiatek MA, Hirano I, Kahrilas PJ, Rothe J, Luger D, Pandolfino JE. Mechanical properties of the esophagus in eosinophilic esophagitis. Gastroenterology. 2011;140(1):82–90.
Larsson H, Norder Grusell E, Tegtmeyer B, Ruth M, Bergquist H, Bove M. Grade of eosinophilia versus symptoms in patients with dysphagia and esophageal eosinophilia. Dis Esophagus. 2016;29(8):971–6.
Nicodème F, Hirano I, Chen J, Robinson K, Lin Z, Xiao Y, Gonsalves N, Kwasny MJ, Kahrilas PJ, Pandolfino JE. Esophageal distensibility as a measure of disease severity in patients with eosinophilic esophagitis. Clin Gastroenterol Hepatol. 2013;11(9):1101–1107.e1.
Byrne KR, Panagiotakis PH, Hilden K, Thomas KL, Peterson KA, Fang JC. Retrospective analysis of esophageal food impaction: differences in etiology by age and gender. Dig Dis Sci. 2007;52(3):717–21.
Lucendo AJ, Friginal-Ruiz AB, Rodríguez B. Boerhaave’s syndrome as the primary manifestation of adult eosinophilic esophagitis. Two case reports and a review of the literature. Dis Esophagus. 2011;24(2):11–5.
Poh CH, Gasiorowska A, Navarro-Rodriguez T, et al. Upper GI tract findings in patients with heartburn in whom proton pump inhibitor treatment failed versus those not receiving antireflux treatment. Gastrointest Endosc. 2010;71(1):28–34.
Foroutan M, Norouzi A, Molaei M, et al. Eosinophilic esophagitis in patients with refractory gastroesophageal reflux disease. Dig Dis Sci. 2010;55(1):28–31. doi:10.1007/s10620-008-0706-z.
Garcia-Compean D, Gonzalez JAG, Garcia CAM, et al. Prevalence of eosinophilic esophagitis in patients with refractory gastroesophageal reflux disease symptoms: a prospective study. Dig Liver Dis. 2011;43(3):204–8.
Spechler SJ, Genta RM, Souza RF. Thoughts on the complex relationship between gastroesophageal reflux disease and eosinophilic esophagitis. Am J Gastroenterol. 2007;102(6):1301–6.
Dellon ES, Gonsalves N, Hirano I, Furuta GT, Liacouras CA, Katzka DA. ACG clinical guideline: evidenced based approach to the diagnosis and management of esophageal eosinophilia and eosinophilic esophagitis (EoE). Am J Gastroenterol. 2013;108(5):679–92 (quiz 693).
Kinoshita Y, Ishimura N, Oshima N, et al. Recent progress in the research of eosinophilic esophagitis and gastroenteritis. Digestion. 2016;93(1):7–12.
Hirashima Y, Kitajima K, Sugi S, et al. Eosinophilic gastroenteritis in the esophagus, stomach, and small intestine in a patient with a choking feeling in the esophagus. Nippon Shokakibyo Gakkai Zasshi. 2007;104:660–5.
Ishimura N, Furuta K, Sato S, Ishihara S, Kinoshita Y. Limited role of allergy testing in patients with eosinophilic gastrointestinal disorders. J Gastroenterol Hepatol. 2013;28(8):1306–13.
Straumann A, Spichtin HP, Grize L, Bucher KA, Beglinger C, Simon HU. Natural history of primary eosinophilic esophagitis: a follow-up of 30 adult patients for up to 11.5 years. Gastroenterology. 2003;125(6):1660–9.
Konikoff MR, Blanchard C, Kirby C, Buckmeier BK, Cohen MB, Heubi JE, Putnam PE, Rothenberg ME. Potential of blood eosinophils, eosinophil-derived neurotoxin, and eotaxin-3 as biomarkers of eosinophilic esophagitis. Clin Gastroenterol Hepatol. 2006;4(11):1328–36.
Roy-Ghanta S, Larosa DF, Katzka DA. Atopic characteristics of adult patients with eosinophilic esophagitis. Clin Gastroenterol Hepatol. 2008;6(5):531–5.
Penfield JD, Lang DM, Goldblum JR, Lopez RFG. The role of allergy evaluation in adults with eosinophilic esophagitis. J Clin Gastroenterol. 2010;44(1):22–7.
Kagalwalla AF, Sentongo TA, Ritz S, et al. Effect of six-food elimination diet on clinical and histologic outcomes in eosinophilic esophagitis. Clin Gastroenterol Hepatol. 2006;4(9):1097–102.
Gonsalves N, Yang GY, Doerfler B, Ritz S, Ditto AM, Hirano I. Elimination diet effectively treats eosinophilic esophagitis in adults: food reintroduction identifies causative factors. Gastroenterology. 2012;142(7):1451–1459.e1.
Molina-Infante J, Arias A, Barrio J, Rodríguez-Sánchez J, Sanchez-Cazalilla M, Lucendo AJ. Four-food group elimination diet for adult eosinophilic esophagitis: a prospective multicenter study. J Allergy Clin Immunol. 2014;134(5):1093–1099.e1.
Kim HP, Vance RB, Shaheen NJ, Dellon ES. The prevalence and diagnostic utility of endoscopic features of eosinophilic esophagitis: a meta-analysis. Clin Gastroenterol Hepatol. 2012;10(9):988–96.
Samadi F, Levine MS, Rubesin SE, Katzka DA, Laufer I. Feline esophagus and gastroesophageal reflux. Am J Roentgenol. 2010;194(4):972–6.
Artul S, Shkara HA, Khoury R, Habib G. Human feline oesophagus. BMJ Case Rep. 2014. doi:10.1136/bcr-2013-203061.
Odze RD. Pathology of eosinophilic esophagitis: what the clinician needs to know. Am J Gastroenterol. 2009;104(2):485–90.
Infante JM, Lamana LF. Eosinophilic esophagitis misdiagnosed twice as esophageal candidiasis. Gastrointest Endosc. 2010;71(2):394–5.
Kanakala V, Lamb CA, Haigh C, Stirling RW, Attwood SE. The diagnosis of primary eosinophilic oesophagitis in adults: missed or misinterpreted? Eur J Gastroenterol Hepatol. 2010;22(7):848–55.
Hirano I, Moy N, Heckman MG, Thomas CS, Gonsalves N, Achem SR. Endoscopic assessment of the oesophageal features of eosinophilic oesophagitis: validation of a novel classification and grading system. Gut. 2013;62(4):489–95.
Mulder DJ, Gander S, Hurlbut DJ, Soboleski DA, Smith RG, Justinich CJ. Multiple squamous hyperplastic-fibrous inflammatory polyps of the oesophagus: a new feature of eosinophilic oesophagitis? J Clin Pathol. 2009;62:845–6. doi:10.1136/jcp.2009.066100.
Gill JA, Shutter J, Brady P. A rare endoscopic feature of eosinophilic esophagitis. Endoscopy. 2011;43:E17.
Potter JW, Saeian K, Staff D, Massey BT, Komorowski RA, Shaker RHW. Eosinophilic esophagitis in adults: an emerging problem with unique esophageal features. Gastrointest Endosc. 2004;59(3):355–61.
Remedios M, Campbell C, Jones DM, Kerlin P. Eosinophilic esophagitis in adults: clinical, endoscopic, histologic findings, and response to treatment with fluticasone propionate. Gastrointest Endosc. 2006;63(1):3–12.
Genevay M, Rubbia-Brandt L, Rougemont AL. Do eosinophil numbers differentiate eosinophilic esophagitis from gastroesophageal reflux disease? Arch Pathol Lab Med. 2010;134(6):815–25.
Peery AF, Cao H, Dominik R, Shaheen NJ, Dellon ES. Variable reliability of endoscopic findings with white-light and narrow-band imaging for patients with suspected eosinophilic esophagitis. Clin Gastroenterol Hepatol. 2011;9(6):475–80.
van Rhijn BD, Warners MJ, Curvers WL, van Lent AU, Bekkali NLTR, Kloek JJ, Bergman JJ, Fockens PBA. Evaluating the endoscopic reference score for eosinophilic esophagitis: moderate to substantial intra- and interobserver reliability. Endoscopy. 2014;46(12):1049–55.
Hirano I. Role of advanced diagnostics for eosinophilic esophagitis. Dig Dis. 2014;32(1–2):78–83.
van Rhijn BD, Verheij J, Smout AJPM, Bredenoord AJ. The endoscopic reference score shows modest accuracy to predict histologic remission in adult patients with eosinophilic esophagitis. Neurogastroenterol Motil. 2016;28(11):1714–22.
Dellon ES, Cotton CC, Gebhart JH, Higgins LL, Beitia R, Woosley JT, Shaheen NJ. Accuracy of the eosinophilic esophagitis endoscopic reference score in diagnosis and determining response to treatment. Clin Gastroenterol Hepatol. 2016;14(1):31–9.
Gonsalves N, Policarpio-Nicolas M, Zhang Q, Rao MS, Hirano I. Histopathologic variability and endoscopic correlates in adults with eosinophilic esophagitis. Gastrointest Endosc. 2006;64(3):313–9.
Matsushita T, Maruyama R, Ishikawa N, et al. The number and distribution of eosinophils in the adult human gastrointestinal tract: a study and comparison of racial and environmental factors. Am J Surg Pathol. 2015;39(4):521–7. doi:10.1097/PAS.0000000000000370.
Kato M, Kephart GM, Talley NJ, Wagner JM, Sarr MG, Bonno M, McGovern TW, Gleich GJ. Eosinophil infiltration and degranulation in normal human tissue. Anat Rec. 1998;252(3):418–25.
Winter HS, Madara JL, Stafford RJ, et al. Intraepithelial eosinophils: anewdiagnostic criterion for refluxesophagitis. Gastroenterology. 1982;83:818–23.
Parfitt JR, Gregor JC, Suskin NG, Jawa HA, Driman DK. Eosinophilic esophagitis in adults: distinguishing features from gastroesophageal reflux disease: a study of 41 patients. Mod Pathol. 2006;19:90–6.
Dellon ES, Aderoju A, Woosley JT, Sandler RS, Shaheen NJ. Variability in diagnostic criteria for eosinophilic esophagitis: a systematic review. Am J Gastroenterol. 2007;102(10):2300–13.
Saffari H, Peterson KA, Fang JC, Teman C, Gleich GJ, Pease LF. Patchy eosinophil distributions in an esophagectomy specimen from a patient with eosinophilic esophagitis: implications for endoscopic biopsy. J Allergy Clin Immunol. 2012;130(3):798–800.
Salek J, Clayton F, Vinson L, et al. Endoscopic appearance and location dictate diagnostic yield of biopsies in eosinophilic oesophagitis. Aliment Pharmacol Ther. 2015;41(12):1288–95.
Straumann A, Spichtin HP, Bucher KA, Heer P, Simon HU. Eosinophilic esophagitis: red on microscopy, white on endoscopy. Digestion. 2004;70(2):109–16.
Okimoto E, Ishimura N, Okada M, et al. Specific locations of linear furrows in patients with esophageal eosinophilia. Dig Endosc. 2017;29(1):49–56.
Ravi K, Talley NJ, Smyrk TC, et al. Low grade esophageal eosinophilia in adults: an unrecognized part of the spectrum of eosinophilic esophagitis? Dig Dis Sci. 2011;56(7):1981–6.
Singla MB, Moawad FJ. An overview of the diagnosis and management of eosinophilic esophagitis. Clin Transl Gastroenterol. 2016;7:e155.
Schoepfer AM, Straumann A, Panczak R, et al. Development and validation of a symptom-based activity index for adults with eosinophilic esophagitis. Gastroenterology. 2014;147(6):1255–1266.e21.
Molina-Infante J, Ferrando-Lamana L, Ripoll C, et al. Esophageal eosinophilic infiltration responds to proton pump inhibition in most adults. Clin Gastroenterol Hepatol. 2011;9(2):110–7.
Alexander JA, Jung KW, Arora AS, et al. Swallowed fluticasone improves histologic but not symptomatic response of adults with eosinophilic esophagitis. Clin Gastroenterol Hepatol. 2012;10(7):742–9.
Arias Á, González-Cervera J, Tenias JM, Lucendo AJ. Efficacy of dietary interventions for inducing histologic remission in patients with eosinophilic esophagitis: a systematic review and meta-analysis. Gastroenterology. 2014;146(7):1639–48.
Hirano I. 2015 David Y. Graham lecture: the first two decades of eosinophilic esophagitis-from acid reflux to food allergy. Am J Gastroenterol. 2016;111(6):770–6.
Ngo P, Furuta GT, Antonioli DA, Fox VL. Eosinophils in the esophagus—peptic or allergic eosinophilic esophagitis? Case series of three patients with esophageal eosinophilia. Am J Gastroenterol. 2006;101(7):1666–70.
Dellon ES, Speck O, Woodward K, et al. Clinical and endoscopic characteristics do not reliably differentiate PPI-responsive esophageal eosinophilia and eosinophilic esophagitis in patients undergoing upper endoscopy: a prospective cohort study. Am J Gastroenterol. 2013;108(12):1854–60.
Molina-Infante J, Bredenoord AJ, Cheng E, et al. Proton pump inhibitor-responsive oesophageal eosinophilia: an entity challenging current diagnostic criteria for eosinophilic oesophagitis. Gut. 2016;65(3):524–31.
Moawad FJ, Veerappan GR, Wong RK. Eosinophilic esophagitis. Dig Dis Sci. 2009;54(9):1818–28.
van Rhijn BD, Weijenborg PW, Verheij J, et al. Proton pump inhibitors partially restore mucosal integrity in patients with proton pump inhibitor-responsive esophageal eosinophilia but not eosinophilic esophagitis. Clin Gastroenterol Hepatol. 2014;12(11):1815–23.
Kedika RR, Souza RF, Spechler SJ. Potential anti-inflammatory effects of proton pump inhibitors: a review and discussion of the clinical implications. Dig Dis Sci. 2009;54(11):2312–7.
Cheng E, Zhang X, Huo X, Yu C, Zhang Q, Wang DH, Spechler SJ, Souza RF. Omeprazole blocks eotaxin-3 expression by oesophageal squamous cells from patients with eosinophilic oesophagitis and GORD. Gut. 2013;62(6):824–32.
Hirano I. Editorial: should patients with suspected eosinophilic esophagitis undergo a therapeutic trial of proton pump inhibition? Am J Gastroenterol. 2013;108(3):373–5.
Lucendo AJ, Arias Á, Molina-Infante J. Efficacy of proton pump inhibitor drugs for inducing clinical and histologic remission in patients with symptomatic esophageal eosinophilia: a systematic review and meta-analysis. Clin Gastroenterol Hepatol. 2016;14(1):13–22.e1.
Wen T, Dellon ES, Moawad FJ, Furuta GT, Aceves SS, Rothenberg ME. Transcriptome analysis of proton pump inhibitor-responsive esophageal eosinophilia reveals proton pump inhibitor-reversible allergic inflammation. J Allergy Clin Immunol. 2015;135(1):187–97.
Jiao D, Ishimura N, Maruyama R, et al. Similarities and differences among eosinophilic esophagitis, proton-pump inhibitor-responsive esophageal eosinophilia, and reflux esophagitis: comparisons of clinical, endoscopic, and histopathological findings in Japanese patients. J Gastroenterol. 2017;52(2):203–10. doi:10.1007/s00535-016-1213-1.
Molina-Infante J, Rodriguez-Sanchez J, Martinek J, et al. Long-term loss of response in proton pump inhibitor-responsive esophageal eosinophilia is uncommon and influenced by CYP2C19 genotype and rhinoconjunctivitis. Am J Gastroenterol. 2015;110(11):1567–75.
Gõmez-Torrijos E, García-Rodríguez R, Castro-Jiménez A, Rodríguez-Sanchez J, Méndez Díaz Y, Molina-Infante J. The efficacy of step-down therapy in adult patients with proton pump inhibitor-responsive oesophageal eosinophilia. Aliment Pharmacol Ther. 2016;43(4):534–40.
Dohil R, Aceves S, Newbury RO. Transient PPI responsive esophageal eosinophilia may be a clinical sub-phenotype of pediatric eosinophilic esophagitis. Dig Dis Sci. 2012;57(5):1413–9.
Aceves SS, Bastian JF, Newbury RO, Dohil R. Oral viscous budesonide: a potential new therapy for eosinophilic esophagitis in children. Am J Gastroenterol. 2007;102(10):2271–9.
Dellon ES, Sheikh A, Speck O, et al. Viscous topical is more effective than nebulized steroid therapy for patients with eosinophilic esophagitis. Gastroenterology. 2012;143(2):321–4.
Dellon ES. Diagnosis and management of eosinophilic esophagitis. Clin Gastroenterol Hepatol. 2012;10(10):1066–78.
Lipka S, Kumar A, Miladinovic B, Richter JE. Systematic review with network meta-analysis: comparative effectiveness of topical steroids vs. PPIs for the treatment of the spectrum of eosinophilic oesophagitis. Aliment Pharmacol Ther. 2016;43(6):663–73.
Sawas T, Dhalla S, Sayyar M, Pasricha PJ, Hernaez R. Systematic review with meta-analysis: pharmacological interventions for eosinophilic oesophagitis. Aliment Pharmacol Ther. 2015;41(9):797–806.
Murali AR, Gupta A, Attar BM, Ravi V, Koduru P. Topical steroids in eosinophilic esophagitis: systematic review and meta-analysis of placebo-controlled randomized clinical trials. J Gastroenterol Hepatol. 2016;31(6):1111–9.
Straumann A, Conus S, Degen L, et al. Long-term budesonide maintenance treatment is partially effective for patients with eosinophilic esophagitis. Clin Gastroenterol Hepatol. 2011;9(5):400–409.e1.
Lucendo AJ, Arias Á, De Rezende LC, et al. Subepithelial collagen deposition, profibrogenic cytokine gene expression, and changes after prolonged fluticasone propionate treatment in adult eosinophilic esophagitis: a prospective study. J Allergy Clin Immunol. 2011;128(5):1037–46.
Chuang MYA, Chinnaratha MA, Hancock DG, et al. Topical steroid therapy for the treatment of eosinophilic esophagitis (EoE): a systematic review and meta-analysis. Clin Transl Gastroenterol. 2015;6(3):e82.
Kuchen T, Straumann A, Safroneeva E, et al. Swallowed topical corticosteroids reduce the risk for long-lasting bolus impactions in eosinophilic esophagitis. Allergy. 2014;69(9):1248–54.
Schaefer ET, Fitzgerald JF, Molleston JP, et al. Comparison of oral prednisone and topical fluticasone in the treatment of eosinophilic esophagitis: a randomized trial in children. Clin Gastroenterol Hepatol. 2008;6(2):165–73.
Helou EF, Simonson J, Arora AS. 3-Yr-follow-up of topical corticosteroid treatment for eosinophilic esophagitis in adults. Am J Gastroenterol. 2008;103(9):2194–9.
Golekoh MC, Hornung LN, Mukkada VA, Khoury JC, Putnam PE, Backeljauw PF. Adrenal insufficiency after chronic swallowed glucocorticoid therapy for eosinophilic esophagitis. J Pediatr. 2016;170:240–5.
Pasha SF, Acosta RD, Chandrasekhara V, et al. The role of endoscopy in the evaluation and management of dysphagia. Gastrointest Endosc. 2014;79(2):191–201.
Ally MR, Dias J, Veerappan GR, Maydonovitch CL, Wong RK, Moawad FJ. Safety of dilation in adults with eosinophilic esophagitis. Dis Esophagus. 2013;26(3):241–5.
Runge TM, Eluri S, Cotton CC, et al. Outcomes of esophageal dilation in eosinophilic esophagitis: safety, efficacy, and persistence of the fibrostenotic phenotype. Am J Gastroenterol. 2016;111(2):206–13.
Bohm ME, Richter JE. Review article: oesophageal dilation in adults with eosinophilic oesophagitis. Aliment Pharmacol Ther. 2011;33(7):748–57.
Jacobs JW, Spechler SJ. A systematic review of the risk of perforation during esophageal dilation for patients with eosinophilic esophagitis. Dig Dis Sci. 2010;55(6):1512–5.
Dellon ES, Gibbs WB, Rubinas TC, et al. Esophageal dilation in eosinophilic esophagitis: safety and predictors of clinical response and complications. Gastrointest Endosc. 2010;71(4):706–12.
Schoepfer AM, Gonsalves N, Bussmann C, et al. Esophageal dilation in eosinophilic esophagitis: effectiveness, safety, and impact on the underlying inflammation. Am J Gastroenterol. 2010;105(5):1062–70.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest:
Yasuhiko Abe, Yu Sasaki, Makoto Yagi, Takao Yaoita, Shoichi Nishise, and Yoshiyuki Ueno have no conflict of interest.
Human Rights:
All procedures followed have been performed in accordance with the ethical standards laid down in the 1964 Declaration of Helsinki and its later amendments.
Informed Consent:
Informed consent was obtained from all patients for being included in the study.
Rights and permissions
About this article
Cite this article
Abe, Y., Sasaki, Y., Yagi, M. et al. Diagnosis and treatment of eosinophilic esophagitis in clinical practice. Clin J Gastroenterol 10, 87–102 (2017). https://doi.org/10.1007/s12328-017-0725-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12328-017-0725-4