Abstract
Introduction
BT-001 (AspyreRx™) prescription digital therapy, a form of personalized cognitive behavioral therapy, has demonstrated clinically meaningful and durable hemoglobin A1c reductions in patients with type 2 diabetes (T2D). The current study examined the cost-effectiveness of BT-001 plus standard of care (SoC) versus SoC alone in T2D over a lifetime horizon from a healthcare payer perspective.
Methods
We modeled the T2D pathway using an individual patient-level simulation; clinical data were sourced from the intention-to-treat subset of the BT-001 randomized clinical trial (RCT). SoC across both arms included the composition of oral and injectable treatments for T2D. Events were simulated using the United Kingdom Prospective Diabetes Study Outcomes Model 2 risk equation. A 3-month model cycle length was used in the first year, then annual model cycles were used in line with the original risk engine specifications. Patient characteristics informed event equations and Monte Carlo random sampling was used to assess the occurrence of events within each model cycle. Incidence of hypoglycemic events, drug discontinuation, costs, and health utilities and disutility values were sourced from the literature.
Results
From a payer perspective, BT-001 plus SoC versus SoC alone was dominant with a gain in quality-adjusted life years (QALYs) of 0.101 and cost savings of $7343 per patient over the lifetime horizon (i.e., more effective and less costly). BT-001 plus SoC was cost-effective at a willingness-to-pay of $100,000 per QALY (incremental net monetary benefit was $17,443). Savings with BT-001 were primarily driven by a reduction in drug acquisition costs. The reduction in hemoglobin A1c with BT-001 was associated with fewer T2D complications.
Conclusions
BT-001 plus SoC was estimated to dominate SoC alone over the lifetime horizon from a payer perspective, suggesting that using BT-001 can empower patients to better manage their diabetes with the potential for lifelong advantages.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Why carry out this study? |
BT-001 has been authorized by the US Food and Drug Administration as a prescription digital therapeutic that uses personalized cognitive behavioral therapy to treat patients with type 2 diabetes (T2D). |
A randomized clinical trial for BT-001 demonstrated significant and durable hemoglobin A1c reductions in T2D. However, data on the impact of digital therapeutics in T2D on economic outcomes is limited. |
It is important to assess both the clinical and economic outcomes of an intervention for decision-making. |
What was learned from this study? |
BT-001 plus standard of care (SoC) was anticipated to dominate SoC alone over the lifetime horizon from a payer perspective, suggesting that using BT-001 can empower patients to better manage their diabetes, with the potential to reduce long-term complications. |
The incremental net monetary benefit of BT-001 plus SoC over SoC alone was $17,443 and considered cost-effective at a willingness-to-pay of $100,000 per quality-adjusted life year. |
Savings with BT-001 were primarily driven by a reduction in drug acquisition costs. |
Introduction
Type 2 diabetes (T2D) is a lifelong and chronic disease and is the most expensive chronic condition in the USA, with both significant direct medical costs and indirect impacts on productivity [1]. More than 28 million Americans have been diagnosed with T2D, and an additional eight million have undiagnosed T2D [2]. At least 45% of patients with T2D fail to achieve adequate glycemic control (hemoglobin A1c [HbA1c] < 7%) with the current standard of care (SoC) options resulting in high rates of morbidity and mortality [3]. There is an unmet need for a novel treatment approach as rates of diabetes continue to rise with a significant economic burden.
In‐person cognitive behavioral therapy (CBT)-based interventions have been shown to improve glycemic control in patients with diabetes [4,5,6]. Internet-based CBT demonstrated equivalent efficacy to in-person CBT, suggesting it is a safe and effective alternative for mental health and psychiatric care applications [7]. Similarly, digital therapeutics may utilize CBT to prevent, manage, and treat chronic disease in a manner similar to the in-person CBT methods utilized for the same conditions, with the major advantage of being easily accessible through one’s smartphone. Improvement in blood glucose levels was illustrated in various racial/ethnic populations with T2D following the use of a digital therapeutic platform [5, 8]. Digital therapeutics have the potential to improve access as they are inherently scalable and more broadly accessible to patients including those lacking transportation, residing in rural areas, and who need childcare or time off work to attend appointments [7].
BT-001 (AspyreRx™) is a prescription-only digital therapeutic (PDT) intended to provide CBT to patients 18 years or older with T2D in 3-month treatment cycles. The PDT treats T2D by targeting the behaviors related to achieving glycemic control in patients who are under the care of a physician and can be used adjunctively with pharmacological diabetes treatments [9]. The safety and efficacy of BT-001 with SoC compared to SoC alone were assessed in an open-label, parallel-group randomized clinical trial (RCT) in 668 patients with T2D. This RCT for BT-001 demonstrated significant and durable HbA1c reductions [8].
It is important to assess both the clinical and economic outcomes of an intervention for decision-making. There is limited evidence on the economic outcomes of digital interventions in T2D. Only one study examined the economic impact of digital behavioral intervention in T2D and suggested that digital interventions may provide substantial cost savings [10]. To fill these knowledge gaps, this study examined the cost-effectiveness of BT-001 plus SoC versus SoC alone in T2D over a lifetime horizon from a healthcare payer perspective.
Methods
Model Overview
The BT-001 model simulated the T2D pathway using an individual patient-level simulation structured to the specifications of the Institute for Clinical and Economic Review (ICER)’s T2D appraisals of semaglutide and tirzepatide [11, 12]. Patient-level simulations estimate outcomes for individual patients; outcomes are then averaged across a sufficiently large sample. Patient-level simulations have commonly been used in T2D [13], offering an advantage over traditional cohort-level modeling because they can account for heterogeneity in the characteristics of individuals and the progression of different diabetes-related complications. The model was built in Microsoft Excel and the model engine was implemented in Visual Basic for Applications.
Model Structure
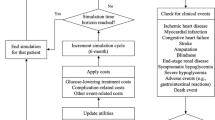
The model structure is presented in Fig. 1. Patient-level data for BT-001 plus SoC versus SoC alone was sourced from the BT-001 RCT [14] and was used to assign patient characteristics at baseline. Patient characteristics included demographics (e.g., age, sex, ethnicity), patient behaviors (e.g., smoking status), disease history (e.g., myocardial infarction, stroke, blindness), and other clinical variables (e.g., HbA1c, systolic blood pressure [SBP], body mass index [BMI], low-density lipoprotein [LDL], duration of diabetes). Events were simulated using the United Kingdom Prospective Diabetes Study (UKPDS) Outcomes Model 2 (OM2) risk equations, which are widely used in diabetes patient-level simulation models [15]. UKPDS OM2 comprises 17 risk equations including seven macrovascular T2D complications (covering congestive heart failure, ischemic heart disease, myocardial infarction, and stroke), six microvascular T2D complications (covering blindness, foot ulcers, amputation, and end-stage renal disease), and four death risk equations [15].
A 3-month model cycle length was used in the first year to capture BT-001-specific costs and clinical inputs, then annual model cycles were used in line with the original risk engine specifications (UKPDS OM2). In each model cycle, patient characteristics informed event equations that predicted the clinical outcomes. The probability of each event was converted to the cycle length and Monte Carlo random sampling was used to assess whether the patient experienced the event or not. Event history was recorded and patient characteristics were updated over time. The clinical experience of a patient was estimated in each cycle, until death was predicted or until the time horizon (lifetime). Outcomes were stored and the next patient was then simulated through the model. After every patient was simulated, results were aggregated as average cost and health outcomes.
Patient Population
The population in the model comprised 610 patients to match the intention-to-treat (ITT) population of the BT-001 RCT. The ITT population included participants who completed onboarding into the assigned treatment and had an HbA1c at the day 90 study time point [14], and was the primary source of demographic and patient history data (Table 1). Variables required for risk estimation that were not collected in the BT-001 RCT were derived from National Health and Nutrition Examination Survey (NHANES, 2017–2020) data, filtered to identify patients with T2D and HbA1c between 7% and 11% (n = 593), in line with the trial population [16].
Random sampling was used to assign patient histories of stroke and ischemic heart disease in the model, based on the proportions derived from NHANES. Patient-reported histories of congestive heart disease in NHANES data [16] were assumed to be the same as histories of ischemic heart disease (National Health Survey, 2020) [17]. Patients with albumin-to-creatinine ratio (ACR) of 30–300 mg/g were assumed to have microalbuminuria and patients with ACR > 300 mg/g to have macroalbuminuria [17, 18]. Mean values for continuous variables in NHANES were assigned to patients in the model for hemoglobin, white blood cell count, and creatinine [16]. All patients entering the model were assumed to have no prior history of blindness, foot ulcer(s), or hypoglycemia, similar to trial exclusion criteria.
This study does not directly involve any human participants, human data, and/or human material. This article is based on previously conducted studies and does not contain any studies with human participants or animals performed by any of the authors.
Treatment Strategies
The model evaluated two treatment strategies: BT-001 plus SoC versus SoC alone, aligning with the randomized treatment arms of the BT-001 RCT.
The SoC offered to both groups in the BT-001 RCT included health education on diet, exercise, and weight loss at the start of the trial. HbA1c and biometric assessments, including blood pressure and weight measurements, were conducted every 3 months at scheduled medical visits, during which medications were adjusted on the basis of the collected values.
SoC was implemented in the model as the composition of oral and injectable treatments received by patients in the RCT. This aligns with the set of treatments to which patients with T2D are usually exposed, including metformin, sulfonylurea, dipeptidyl peptidase 4 (DPP4), glucagon-like peptide 1 (GLP-1), and insulin. The composition of treatments was set to vary by treatment arm over time, in line with the observed distributions in the BT-001 RCT (Supplementary Table 1). In the model, a patient was initiated onto an insulin regimen if their HbA1c exceeded a specific threshold, and could discontinue insulin if their HbA1c level dropped below a specific threshold.
All patients in the model were routed through both intervention and comparator arms, using common random numbers to allow treatments to be compared under “similar experimental conditions” [19]. This ensured that the same patient attributes were sampled across arms, and event risks were equal, ceteris paribus.
Clinical Inputs
Inputs for the BT-001 model were based on modeling precedent set by ICER [11, 12].
Treatment-Related Efficacy
Mean changes in HbA1c, weight, SBP, and Patient Health Questionnaire-9 (PHQ-9) at day 90 and day 180 from the BT-001 RCT were used to define short-term efficacy (Table 2) [14]. Beyond the first year, time-varying risk factors including HbA1c, SBP, LDL, body weight, smoking status, and occurrence of severe hypoglycemia and symptomatic hypoglycemia were modeled using published equations [20].
Incidence of Hypoglycemia Events
Hypoglycemia events were evaluated in the model after year 1 and sourced from published literature [21]. Among patients not yet receiving insulin, the annual probability of severe and mild or moderate hypoglycemic events was 5% and 33%, respectively [21]. Among patients receiving insulin, the annual probability of severe and mild or moderate hypoglycemic events was 21% and 52%, respectively [21].
Depression
The UKPDS OM2 risk engine focuses on physical domains of health. Recognizing the impact of diabetes management on mental well-being, we have included depression as a considered complication in the simulation. In the first year of the model, PHQ-9 data from the BT-001 RCT were used to determine depression status. Beyond year 1, the annual probability of remaining depressed was 36%, calculated from the 4-month probability of 71% reported in the published literature [22]. The annual probability of becoming depressed was 1.20%, sourced from the published literature [23].
Drug Discontinuation
The annual drug discontinuation rate was 9.10% and the HbA1c threshold to commence insulin was greater than or equal to 8.50%. Estimates for both parameters were sourced from an ICER (2022) report on tirzepatide (EMPA-REG EXTEND trial) [12].
Cost Inputs
The model accounted for direct costs related to treatment, monitoring, adverse events, and resource utilization (Table 3). All costs were adjusted to 2022 US dollars and inflated using the Consumer Price Index [24], where required.
Drug Acquisition Costs
The total cost for BT-001 was estimated for 3 months and varied by course of treatment. It was assumed patients can only be on BT-001 in the first 6 months to match the trial data. Net annual insulin acquisition cost was derived from the Red Book [25] and adjusted using patient weight where necessary. Net annual total SoC costs for each drug class were derived on the basis of the wholesale acquisition cost of each drug within each class (from the Red Book [25]), dosing (sourced from prescribing information for each drug), and frequency of use in patients with T2D (from the BT-001 RCT [14]) (Supplementary Tables 1 and 2).
Annual Monitoring Costs
Annual monitoring costs included self-monitoring costs for non-insulin medications and insulin. These costs were sourced from Laiteerapong et al.’s (2018) study that used data from the NHANES 2011–2012 for estimating self-monitoring costs among patients with T2D [21].
Adverse Event Costs
As per the ICER models of semaglutide and tirzepatide [11, 12], complication costs in the year of the event reflected acute care and any subsequent care provided in the first year. Complication costs in the years following the event reflected ongoing maintenance costs. The acute and ongoing annual costs for each adverse event were sourced from the published literature [12, 26, 27]. Ward et al. (2014) used direct data analysis and a micro-costing approach to estimate the costs for an event leading to either a hospital admission or outpatient care and the post-acute care associated with managing macrovascular and microvascular complications, hypoglycemia episodes, and infections [26]. Data were obtained from multiple sources, including national physician and laboratory fee schedules, inpatient and emergency department databases, government reports, and literature [26]. Yang et al. (2020) estimated complication cost both in years of the first occurrence and subsequent years using longitudinal panel data from one of the largest claims databases in the USA for privately insured patients with T1D and T2D with 1 to 10 years of follow-up time [27].
Other Health Conditions
Depression-related costs were also considered in the model and were sourced from Egede et al.’s (2016) study that used data from the 2004–2011 Medical Expenditure Panel Survey (MEPS) to compute nationally representative estimates in adults with diabetes and comorbid depression [28].
Resource Utilization and Costs
Costs for resource utilization included annual outpatient visits (insulin and non-insulin) as well as hypoglycemia episodes requiring hospitalization, emergency department visit, or glucagon injection. Annual outpatient visits were assumed to be required in both arms and related costs were sourced from Laiteerapong et al. (2018) [21]. Estimates on the frequency of hypoglycemia episodes were sourced from Laiteerapong et al. (2018) [21] and related costs were sourced from Ward et al. (2014) [26].
Health Utilities
Utility values are used to represent the quality of life for patients in a specific health state, often ranging between 0 (death) and 1 (perfect health) [29, 30]. Utility values sourced from the published literature were consistent with those used in the ICER reviews (Table 4) [11, 12]. Baseline T2D utility was sourced from Shao et al.’s (2019) study that utilized data from the Action to Control Cardiovascular Risk in Diabetes (ACCORD) trial for generating utilities [31]. The ACCORD trial was one of the largest multicenter trials conducted in patients with T2D from the USA (n = 10,251).
Disutility values were applied additively for patient demographics, injection, and complications. The annual disutility of injection was sourced from Boye et al. (2011), a study on patients with T2D in Scotland that used a standard gamble approach to assess the utility of hypothetical health states and their current health state [32]. Disutility for patient demographics and macrovascular/microvascular complications was sourced from Shao et al. (2019) [31]. Disutility for microvascular complications including foot ulcer and amputation was sourced from Sullivan et al.’s (2016) study that mapped the European Quality of Life 5 Dimensions 3 Level Version (EQ-5D-3L) questionnaire responses from short-form 12 health survey responses in MEPS (2000–2011) data [33].
Model Outcomes
Model results included the occurrence of macrovascular and microvascular complications, severe hypoglycemia, and depression over a lifetime horizon for each treatment arm. Total costs, life years (LYs), and quality-adjusted life years (QALYs) were calculated to support the estimation of cost-effectiveness. Quality of life was modeled with QALYs using projected patient survival weighted by additive disutility values for each diabetes-related complication experienced in each model cycle. The willingness-to-pay threshold for incremental net monetary benefit (INMB) calculations was assumed to be $100,000 per QALY. Cost and health outcomes were discounted annually by 3% [34].
Sensitivity Analyses
In the base case of the model, the point estimate of each input was used to generate results. To account for the uncertainty around model parameters, and to test the robustness of the model, both one-way deterministic sensitivity analysis (DSA) and probabilistic sensitivity analysis (PSA) were conducted. The bounds for DSA were defined using 95% confidence intervals. When there was no uncertainty information for a given parameter, the standard error was assumed to be 10% of the mean estimate and used to estimate lower and upper bounds. In PSA, parameters were represented as distributions around the point estimate and the set of inputs was drawn by random sampling from each distribution. Beta distributions were used for inputs bounded by 0 and 1; gamma distributions were used for costs to account for their common skewness and to ensure non-negativity; and normal distributions were used for efficacy changes from baseline. Results were simulated 200 times per patient, after which cost-effectiveness outcomes had stabilized, resulting in 122,000 effective patients.
Scenario Analyses
In the base case of the model, a lifetime time horizon was assumed (which follows patients from the treatment initiation until age 100 or death); a 3% annual discount rate for costs and health outcomes was applied [34]; patient HbA1c after 1 year was calculated using the Building, Relating, Assessing, and Validating Outcomes (BRAVO) HbA1c time-varying equation [20]; the UKPDS OM2 risk equations were used for complication risks [15]; a healthcare payer perspective was assumed; and depression was modeled over the short term according to PHQ-9 data from the BT-001 RCT and over the long term using published probabilities [22, 23].
Six sets of scenarios were examined. Parameters varied for scenario analysis included (i) a different time horizon (1, 2, 5, 10, and 20 years); (ii) different discounting for costs and benefits (0% and 6%); (iii) considering HbA1c constant after 1 year; (iv) use of BRAVO equations for all complication risks; (v) modified societal perspective (productivity saving per incremental QALY of $5842) [12]; and (vi) exclusion of depression.
Results
Base Case Analysis
Cost-effectiveness Outcomes
Over a lifetime horizon, BT-001 plus SoC was superior to SoC alone in terms of LYs and QALYs per patient (Table 5). Through its impact on HbA1c, BT-001 plus SoC provided 0.034 more LYs than SoC alone, and offered improvements in quality of life with an additional 0.101 QALYs. BT-001 plus SoC versus SoC alone resulted in a cost savings of $7343. At a willingness-to-pay of $100,000 per QALY, BT-001 plus SoC is considered cost-effective over SoC alone with an INMB of $17,443. At other commonly applied thresholds of $50,000 and $150,000 per QALY, the base case inputs yielded INMBs of $12,393 and $22,493, respectively.
Total Costs
The costs were lower for BT-001 plus SoC ($251,148) versus SoC alone ($258,491) over the lifetime horizon (Table 6). Savings with BT-001 were primarily driven by a reduction in drug acquisition costs ($6230) followed by adverse event costs ($438).
Other Outcomes and Adverse Events
A greater proportion of patients experienced better outcomes with BT-001 plus SoC (Table 7). Incremental increases in good HbA1c control (< 8%) and SBP control (≤ 120 mm g) were 12.95% and 9.02%, respectively. The reduction in HbA1c for patients treated with BT-001 plus SoC was associated with fewer T2D macrovascular and microvascular complications (Table 8).
Sensitivity Analysis
DSA
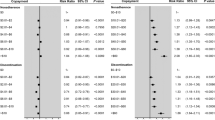
Changes in efficacy (HbA1c and SBP) at 6 months were a key driver of cost-effectiveness (Fig. 2). The change in SoC treatment composition in either arm at the exit visit (day 180+) was also shown in DSA to be influential on results, as drug acquisition costs were impacted. BT-001 remained dominant (cost-saving and more effective) in all cases.
Deterministic sensitivity analysis tornado plot. GLP-1 glucagon-like peptide 1; HbA1c hemoglobin A1c; INMB incremental net monetary benefit; QALY quality-adjusted life years; SBP systolic blood pressure; SGLT2 sodium-glucose cotransporter 2; SoC standard of care. Note: Deterministic sensitivity analysis was performed using one iteration per patient because of its computational intensity, and so results provide an indication of directionality, rather than a full assessment
PSA
The average results generated in PSA aligned with the deterministic base case (Fig. 3). On average, BT-001 use was associated with incremental QALYs of 0.102 and savings of $7192. At the a priori willingness-to-pay threshold of $100,000 per QALY, BT-001 was cost-effective in 100% of iterations, and this held true at a $50,000 per QALY threshold. BT-001 was cost-saving in 98.0% of iterations. The INMB was stabilized beyond 150 iterations (Supplementary Fig. 1).
Scenario Analyses
At shorter time horizons of 1, 2, and 5 years, cost-effectiveness was less favorable compared with a longer time horizon (Fig. 4). The most favorable scenario was 0% discounting for costs and benefits, followed by assuming constant HbA1c after 1 year of follow-up, and then BRAVO risk equations.
Discussion
Through its impact on HbA1c and other variables, BT-001 was associated with greater LYs, QALYs, and lower costs over a lifetime horizon. BT-001 plus SoC was shown to be more effective and less costly than SoC alone. Savings with BT-001 were primarily driven by a reduction in drug acquisition costs of SoC (insulin and non-insulin) treatments. While other cost categories were significant contributors, the higher impact of changes in acquisition costs is largely due to the inherent expensiveness of certain T2D medications, which were mitigated by the implementation of BT-001. BT-001 provided better HbA1c and SBP control, delaying the escalation to insulin. T2D complications were also reduced leading to lower costs associated with adverse events.
The cost-effectiveness of prescription digital therapy in T2D has been examined in a limited number of studies [10]. A decision analytic model from a US commercial payer perspective was used to examine the economic impact of digital behavioral therapy in T2D and hypertension [10]. Over a 3-year time horizon, the average health resource utilization savings ranged from $97 to $145 per patient per month. Using a willingness-to-pay threshold of $100,000/QALY, digital therapy in T2D was estimated to be cost-effective at total 3-year program costs of $8348. These estimates are in line with our findings with lifetime cost savings of $7343 following the usage of BT-001. Both, the study and our findings, describe the potential reduction in medication costs to be the primary driver in cost savings [10].
Changes in efficacy at 6 months and changes in treatment composition at the exit visit were also key drivers of cost-effectiveness in this model. At 1 year, a greater proportion of patients experienced better SBP outcomes with BT-001 (50.7% vs 41.6%), an important outcome for payers. Furthermore, BT-001 remained dominant in several sensitivity analyses. At shorter time horizons of 1, 2, and 5 years, there was less opportunity for the lifetime advantages of BT-001 to occur and treatment was less cost-effective.
The model included all treatment costs associated with each drug regimen, including acquisition costs for SoC, cyclic costs of BT-001, monitoring costs, resource use costs, and all costs associated with diabetes-related complications experienced in each model cycle. The increases in depression and end-stage renal disease (ESRD; microvascular complication) rates for the BT-001 plus SoC arm were driven solely by the extension in survival time. The associated costs only increased for ESRD by $581.
This cost-effectiveness analysis follows the modeling precedent established by ICER [11, 12]. Digital therapeutics using behavioral interventions have the potential to improve access owing to their inherent scalability and reach beyond physical location and scheduling constraints. App-based healthcare can empower patients to develop healthy behaviors leading to better adherence and lower costs. A recent study examined the impact of a digital Diabetes Prevention Program among 2027 adult participants and demonstrated a reduction in all-cause health care spending of USD 1169 per participant owing to fewer hospital admissions and shorter lengths of stay [36]. App-based healthcare interventions have demonstrated cost-effectiveness in T2D and other therapeutic areas.
At the time of writing, BT-001 has not yet been studied beyond 180 days. The instructions for use indicate that a second 90-day treatment could be prescribed and is likely to offer further benefit to the patient. It should be acknowledged that if further courses are administered in clinical practice beyond 180 days, additional acquisition costs for BT-001 would be incurred. As BT-001 promotes long-lasting changes in the underlying core beliefs related to diabetes management behaviors, it is plausible that further treatment offers further benefits to the patient, which could offset this additional cost.
The results from this study should be interpreted carefully with the following limitations in mind. First, only short-term data was available from the BT-001 RCT and may have resulted in under- or overestimation of long-term clinical and economic outcomes. Second, clinical data from the BT-001 RCT may limit the application of these findings to real-world clinical practice. Patients in the BT-001 RCT were well treated (e.g., baseline SBP was near normal, very high use of background therapy) and the SoC treatment may have underestimated effect sizes by introducing a degree of glycemic equipoise not likely observed in the real world. However, the BT-001 RCT had many real-world elements. Most notably the ability for medications to be adjusted from day 1, open-label draws of HbA1c, and no compensation was provided for use of cognitive behavioral therapy (CBT) features. On balance, the use of randomized controlled evidence reduces the potential for selection bias (differences in confounding factors at baseline) associated with real-world evidence. A third limitation is that medical history was self-reported, which may lead to underestimation of the prevalence of baseline comorbidities. The model had a reliance on certain assumptions; however, sensitivity analyses were conducted to overcome this limitation and test uncertainty with specific parameters. Finally, the complexity of T2D renders it challenging to make reliable predictions, although the model considered patient characteristics, comorbidities, and risk equations to account for this heterogeneity.
Conclusions
This model found that BT-001 plus SoC dominated over SoC alone over the lifetime horizon from a payer perspective, suggesting that BT-001 can empower patients to better manage their diabetes, with the potential for lifelong advantages.
Data Availability
Data sharing does not apply to this article as no datasets were generated or analyzed during the current study.
References
American Diabetes Association. Economic costs of diabetes in the U.S. in 2017. Diabetes Care. 2018;41(5):917–28. https://doi.org/10.2337/dci18-0007.
Centers for Disease Control and Prevention. National Diabetes Statistics Report. https://www.cdc.gov/diabetes/data/statistics-report/index.html. Accessed 10 Oct 2023.
Polonsky WH, Henry RR. Poor medication adherence in type 2 diabetes: recognizing the scope of the problem and its key contributors. Patient Prefer Adher. 2016;10:1299–307. https://doi.org/10.2147/PPA.S106821.
Chapman A, Liu S, Merkouris S, et al. Psychological interventions for the management of glycemic and psychological outcomes of type 2 diabetes mellitus in china: a systematic review and meta-analyses of randomized controlled trials. Front Public Health. 2015;3:252. https://doi.org/10.3389/fpubh.2015.00252.
Gershoni T, Ritholz MD, Horwitz DL. Glycemic management by a digital therapeutic platform across racial/ethnic groups: a retrospective cohort study. Appl Sci. 2023. https://doi.org/10.3390/app13010431.
Yang X, Li Z, Sun J. Effects of cognitive behavioral therapy-based intervention on improving glycaemic, psychological, and physiological outcomes in adult patients with diabetes mellitus: a meta-analysis of randomized controlled trials. Front Psychiatry. 2020;11:711. https://doi.org/10.3389/fpsyt.2020.00711.
Wilhelm S, Weingarden H, Ladis I, Braddick V, Shin J, Jacobson NC. Cognitive-behavioral therapy in the digital age: presidential address. Behav Ther. 2020;51(1):1–14. https://doi.org/10.1016/j.beth.2019.08.001.
Hsia J, Guthrie NL, Lupinacci P, et al. Randomized, controlled trial of a digital behavioral therapeutic application to improve glycemic control in adults with type 2 diabetes. Diabetes Care. 2022;45(12):2976–81. https://doi.org/10.2337/dc22-1099.
US Food & Drug Administration. FDA Correspondence Letter DEN220058 - BT-001. https://www.accessdata.fda.gov/cdrh_docs/pdf22/DEN220058.pdf. Accessed 25 Oct 2023.
Nordyke RJ, Appelbaum K, Berman MA. Estimating the impact of novel digital therapeutics in type 2 diabetes and hypertension: health economic analysis. J Med Internet Res. 2019;21(10):e15814. https://doi.org/10.2196/15814.
Institute for Clinical and Economic Review. Oral semaglutide for type 2 diabetes: effectiveness and value. 2019. https://icer.org/wp-content/uploads/2020/10/ICER_Diabetes_Final-Evidence-Report_120919-1.pdf. Accessed 14 June 2023.
Institute for Clinical and Economic Review. Tirzepatide for type 2 diabetes: Final Report. 2022. https://icer.org/wp-content/uploads/2021/06/ICER_Type-2-Diabetes_12-Month_Check-Up_03312023.pdf. Accessed 14 June 2023.
Li J, Bao Y, Chen X, Tian L. Decision models in type 2 diabetes mellitus: a systematic review. Acta Diabetol. 2021;58(11):1451–69. https://doi.org/10.1007/s00592-021-01742-6.
Canonico ME, Hsia J, Guthrie NL, et al. Cognitive behavioral therapy delivered via digital mobile application for the treatment of type 2 diabetes: rationale, design, and baseline characteristics of a randomized, controlled trial. Clin Cardiol. 2022;45(8):850–6. https://doi.org/10.1002/clc.23853.
Hayes AJ, Leal J, Gray AM, Holman RR, Clarke PM. UKPDS outcomes model 2: a new version of a model to simulate lifetime health outcomes of patients with type 2 diabetes mellitus using data from the 30 year United Kingdom Prospective Diabetes Study: UKPDS 82. Diabetologia. 2013;56(9):1925–33. https://doi.org/10.1007/s00125-013-2940-y.
National Health and Nutrition Examination Survey. NHANES 2017-March 2020 Pre-pandemic data. https://wwwn.cdc.gov/nchs/nhanes/continuousnhanes/default.aspx?cycle=2017-2020. Accessed May 2022.
National Health Service. Overview - Coronary heart disease. https://www.nhs.uk/conditions/coronary-heart-disease. Accessed May 2022.
National Kidney Foundation. ACR. https://www.kidney.org/kidneydisease/siemens_hcp_acr. Accessed May 2022.
National Institute for Health and Care Excellence. Cost-effectiveness modelling using patient-level simulation. https://www.sheffield.ac.uk/nice-dsu/tsds/patient-level-simulation. Accessed 14 June 2023.
Shao H, Fonseca V, Stoecker C, Liu S, Shi L. Novel risk engine for diabetes progression and mortality in USA: Building, Relating, Assessing, and Validating Outcomes (BRAVO). Pharmacoeconomics. 2018;36(9):1125–34. https://doi.org/10.1007/s40273-018-0662-1.
Laiteerapong N, Cooper JM, Skandari MR, et al. Individualized glycemic control for US adults with type 2 diabetes: a cost-effectiveness analysis. Ann Intern Med. 2018;168(3):170–8. https://doi.org/10.7326/M17-0537.
Camacho EM, Ntais D, Coventry P, et al. Long-term cost-effectiveness of collaborative care (vs usual care) for people with depression and comorbid diabetes or cardiovascular disease: a Markov model informed by the COINCIDE randomised controlled trial. BMJ Open. 2016;6(10):e012514. https://doi.org/10.1136/bmjopen-2016-012514.
Salinero-Fort MA, Gomez-Campelo P, San Andres-Rebollo FJ, et al. Prevalence of depression in patients with type 2 diabetes mellitus in Spain (the DIADEMA Study): results from the MADIABETES cohort. BMJ Open. 2018;8(9):e020768. https://doi.org/10.1136/bmjopen-2017-020768.
United States Bureau of Labor Statistics. Databases, Tables & Calculators by Subject. https://data.bls.gov/timeseries/CUUR0000SAM2. Accessed 11 May 2022.
Truven Health Analytics. Red Book Online Search. http://www.micromedexsolutions.com. Accessed 13 Nov 2022.
Ward A, Alvarez P, Vo L, Martin S. Direct medical costs of complications of diabetes in the United States: estimates for event-year and annual state costs (USD 2012). J Med Econ. 2014;17(3):176–83. https://doi.org/10.3111/13696998.2014.882843.
Yang W, Cintina I, Hoerger T, et al. Estimating costs of diabetes complications in people < 65 years in the US using panel data. J Diabetes Complicat. 2020;34(12):107735. https://doi.org/10.1016/j.jdiacomp.2020.107735.
Egede LE, Walker RJ, Bishu K, Dismuke CE. Trends in costs of depression in adults with diabetes in the United States: medical expenditure panel survey, 2004–2011. J Gen Intern Med. 2016;31(6):615–22. https://doi.org/10.1007/s11606-016-3650-1.
Weinstein MC, Torrance G, McGuire A. QALYs: the basics. Value Health. 2009;12(Suppl 1):S5-9. https://doi.org/10.1111/j.1524-4733.2009.00515.x.
Bakker C, van der Linden S. Health related utility measurement: an introduction. J Rheumatol. 1995;22(6):1197–9.
Shao H, Yang S, Fonseca V, Stoecker C, Shi L. Estimating quality of life decrements due to diabetes complications in the United States: the health utility index (HUI) diabetes complication equation. Pharmacoeconomics. 2019;37(7):921–9. https://doi.org/10.1007/s40273-019-00775-8.
Boye KS, Matza LS, Walter KN, Van Brunt K, Palsgrove AC, Tynan A. Utilities and disutilities for attributes of injectable treatments for type 2 diabetes. Eur J Health Econ. 2011;12(3):219–30. https://doi.org/10.1007/s10198-010-0224-8.
Sullivan PW, Ghushchyan VH. EQ-5D scores for diabetes-related comorbidities. Value Health. 2016;19(8):1002–8. https://doi.org/10.1016/j.jval.2016.05.018.
Institute for Clinical and Economic Review. ICER’s reference case for economic evaluations: principles and rationale. https://icer.org/wp-content/uploads/2020/10/ICER_Reference_Case_013120.pdf. Accessed 14 June 2023.
Wexler DJ, Grant RW, Wittenberg E, et al. Correlates of health-related quality of life in type 2 diabetes. Diabetologia. 2006;49(7):1489–97. https://doi.org/10.1007/s00125-006-0249-9.
Sweet CC, Jasik CB, Diebold A, DuPuis A, Jendretzke B. Cost savings and reduced health care utilization associated with participation in a digital diabetes prevention program in an adult workforce population. J Health Econ Outcomes Res. 2020;7(2):139–47. https://doi.org/10.36469/jheor.2020.14529.
Acknowledgements
Medical Writing, Editorial, and Other Assistance
Medical writing assistance was provided by Dr. Ishveen Chopra. Support for this assistance was funded by Better Therapeutics.
Authorship
All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this article, take responsibility for the integrity of the work as a whole, and have given their approval for this version to be published.
Funding
This study and the journal’s Rapid Service and Open Access Fees were funded by Better Therapeutics.
Author information
Authors and Affiliations
Contributions
Model conceptualization: NJD, SM, RJN; Model development: NJD, SM; Data analysis: NJD, SM, NLG, PL; Critical review of model: NLG, PL, RJN, MAB; Writing—review and editing: All authors.
Corresponding author
Ethics declarations
Conflict of Interest
Niall J. Davison and Sarah Medland were contracted consultants of Maple Health Group, who were paid for work related to the model and manuscript by the study sponsor (Better Therapeutics). Sarah Medland’s current affiliation is York Health Economics Consortium, United Kingdom. Nicole L. Guthrie and Mark A. Berman were employees of Better Therapeutics at the time of the study and received compensation in the form of equity units and stock options. Paul Lupinacci was a contracted consultant of the study sponsor (Better Therapeutics) and was paid for work related to the manuscript. Robert J. Nordyke has no conflicts of interest to declare.
Ethical Approval
This study does not directly involve any human participants, human data, and/or human material. This article is based on previously conducted studies and does not contain any studies with human participants or animals performed by any of the authors.
Additional information
This manuscript is based on the results of the BT-001 randomized controlled trial, which is detailed in Hsia et al. (Diabetes Care 45(12):2976–2981, 2022).
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Davison, N.J., Guthrie, N.L., Medland, S. et al. Cost-Effectiveness Analysis of a Prescription Digital Therapeutic in Type 2 Diabetes. Adv Ther 41, 806–825 (2024). https://doi.org/10.1007/s12325-023-02752-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12325-023-02752-2