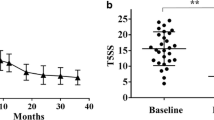
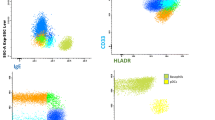
Abstract
Introduction
Our prior clinical study assessed the efficacy and safety of sublingual immunotherapy (SLIT) with standardized Dermatophagoides farina drops on patients with allergic rhinitis (AR) while analyzing the characteristics of adverse reactions. This study was conducted to evaluate the immune cell composition alterations in AR patients before and after SLIT, and to comprehensively investigate the role and changes of antigen-specific immune cells associated with treatment efficacy.
Methods
A total of 68 AR patients who completed 12 months of SLIT were included in the study. Before the trial’s initiation and after 1 year of SLIT, 10 ml of venous blood was collected. Peripheral blood mononuclear cells were isolated using the Ficoll gradient method. The mRNA transcriptome was analyzed using an Affymetrix microarray. The proportions of 22 immune cell types were calculated via the CIBERSORTx platform. Correlations between each immune cell type and SLIT were analyzed. PI3K-PKB pathway dysregulation were analyzed using quantitative PCR and Western blot. Flow cytometry was utilized to assess the percentages of Th1 and Th2 cells.
Results
Mono-sensitized AR patients exhibited marked increases in plasma cells, activated memory T cells, regulatory T cells, and activated dendritic cells, while experiencing decreased neutrophils and resting dendritic cells. In poly-sensitized AR patients, the most notable change was an increase in regulatory T cells, coupled with decreased T follicular helper cells, resting dendritic cells, and activated mast cells. These findings indicated that SLIT reshaped immune cell profiles in AR patients, and, notably, the specific changes differed between mono-sensitized and poly-sensitized individuals. Furthermore, SLIT appeared to shift the immune response towards a Th2 decrease profile in both groups. Importantly, suppression of the PI3K-PKB pathway was evidenced as inhibition of PKB phosphorylation and the decrease of glycogen synthase kinase 3 β (GSKβ) and mammalian target of rapamycin (mTOR) expression after SLIT.
Conclusion
Our study has demonstrated that SLIT treatment led to distinct changes in immune cell profiles between mono-sensitized and poly-sensitized AR patients. Furthermore, SLIT appeared to reduce a Th2 immune response, highlighting its efficacy in AR treatment. Importantly, the study revealed the suppression of the PI3K-PKB pathway, shedding light on the immunological mechanisms underlying SLIT’s effectiveness.






Similar content being viewed by others
Data Availability
The datasets during and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
Bousquet J, Van Cauwenberge P, Khaltaev N, Aria Workshop G, World Health O. Allergic rhinitis and its impact on asthma. J Allergy Clin Immunol. 2001;108:S147-334. https://doi.org/10.1067/mai.2001.118891.
Cox LS, Larenas Linnemann D, Nolte H, Weldon D, Finegold I, Nelson HS. Sublingual immunotherapy: a comprehensive review. J Allergy Clin Immunol. 2006;117:1021–35. https://doi.org/10.1016/j.jaci.2006.02.040.
Maloney J, Bernstein DI, Nelson H, Creticos P, Hebert J, Noonan M, Skoner D, Zhou Y, Kaur A, Nolte H. Efficacy and safety of grass sublingual immunotherapy tablet, MK-7243: a large randomized controlled trial. Ann Allergy Asthma Immunol. 2014;112(146–53): e2. https://doi.org/10.1016/j.anai.2013.11.018.
Ma X, Zhang Y, Gu X, Wu G, Liu J, Lu J, Yang H. A retrospective cohort study of sublingual immunotherapy with standardized dermatophagoides farinae drops for allergic rhinitis. Adv Ther. 2021;38:2315–22. https://doi.org/10.1007/s12325-021-01686-x.
Bousquet J, Lockey R, Malling HJ. Allergen immunotherapy: therapeutic vaccines for allergic diseases. A WHO position paper. J Allergy Clin Immunol. 1998;102:558–62. https://doi.org/10.1016/s0091-6749(98)70271-4.
Moingeon P, Batard T, Fadel R, Frati F, Sieber J, Van Overtvelt L. Immune mechanisms of allergen-specific sublingual immunotherapy. Allergy. 2006;61:151–65. https://doi.org/10.1111/j.1398-9995.2006.01002.x.
Durham SR, Till SJ. Immunologic changes associated with allergen immunotherapy. J Allergy Clin Immunol. 1998;102:157–64. https://doi.org/10.1016/s0091-6749(98)70079-x.
Francis JN, James LK, Paraskevopoulos G, Wong C, Calderon MA, Durham SR, Till SJ. Grass pollen immunotherapy: IL-10 induction and suppression of late responses precedes IgG4 inhibitory antibody activity. J Allergy Clin Immunol. 2008;121(1120–5): e2. https://doi.org/10.1016/j.jaci.2008.01.072.
Kim JH, Yoon MG, Seo DH, Kim BS, Ban GY, Ye YM, Shin YS, Park HS. Detection of allergen specific antibodies from nasal secretion of allergic rhinitis patients. Allergy Asthma Immunol Res. 2016;8:329–37. https://doi.org/10.4168/aair.2016.8.4.329.
Jones SM, Kim EH, Nadeau KC, Nowak-Wegrzyn A, Wood RA, Sampson HA, Scurlock AM, Chinthrajah S, Wang J, Pesek RD, Sindher SB, Kulis M, Johnson J, et al. Efficacy and safety of oral immunotherapy in children aged 1–3 years with peanut allergy (the Immune Tolerance Network IMPACT trial): a randomised placebo-controlled study. Lancet. 2022;399:359–71. https://doi.org/10.1016/S0140-6736(21)02390-4.
Newman AM, Liu CL, Green MR, Gentles AJ, Feng W, Xu Y, Hoang CD, Diehn M, Alizadeh AA. Robust enumeration of cell subsets from tissue expression profiles. Nat Methods. 2015;12:453–7. https://doi.org/10.1038/nmeth.3337.
Iinuma T, Okamoto Y, Morimoto Y, Arai T, Sakurai T, Yonekura S, Sakurai D, Hirahara K, Nakayama T. Pathogenicity of memory Th2 cells is linked to stage of allergic rhinitis. Allergy. 2018;73:479–89. https://doi.org/10.1111/all.13295.
Wu G, Zhu H, Wu X, Liu L, Ma X, Yuan Y, Fu X, Zhang L, Lv Y, Li D, Liu J, Lu J, Yu Y, et al. Anti-allergic function of alpha-Tocopherol is mediated by suppression of PI3K-PKB activity in mast cells in mouse model of allergic rhinitis. Allergol Immunopathol (Madr). 2020;48:395–400. https://doi.org/10.1016/j.aller.2019.11.005.
Zhang Y, Zhang Q, Wu X, Wu G, Ma X, Cheng L. Bicalutamide, an androgen receptor antagonist, effectively alleviate allergic rhinitis via suppression of PI3K-PKB activity. Eur Arch Otorhinolaryngol. 2023;280:703–11. https://doi.org/10.1007/s00405-022-07538-w.
Cheng L, Zhou WC. Sublingual immunotherapy of house dust mite respiratory allergy in China. Allergol Immunopathol (Madr). 2019;47:85–9. https://doi.org/10.1016/j.aller.2018.02.008.
Cui L, Li J, Li Y, Xia Z. Long-term efficacy of sublingual mite immunotherapy in monosensitized and polysensitized children with allergic rhinitis: a 7-year prospective study. Int Arch Allergy Immunol. 2019;180:144–9. https://doi.org/10.1159/000500524.
Lin Z, Liu Q, Li T, Chen D, Chen D, Xu R. The effects of house dust mite sublingual immunotherapy in patients with allergic rhinitis according to duration. Int Forum Allergy Rhinol. 2016;6:82–7. https://doi.org/10.1002/alr.21657.
Subspecialty Group of Rhinology EBoCJoOH, Neck S, Subspecialty Group of Rhinology SoOH, Neck Surgery CMA. [Chinese guidelines for diagnosis and treatment of allergic rhinitis]. Zhonghua Er Bi Yan Hou Tou Jing Wai Ke Za Zhi. 2016;51:6–24. https://doi.org/10.3760/cma.j.issn.1673-0860.2016.01.004.
Wang C, Wang K, Liu S, Qin X, Chen K, Zhang T. Decreased level of osteopontin in children with allergic rhinitis during sublingual immunotherapy. Int J Pediatr Otorhinolaryngol. 2016;81:15–20. https://doi.org/10.1016/j.ijporl.2015.12.001.
Zhang Y, Zhang L. Increasing prevalence of allergic rhinitis in China. Allergy Asthma Immunol Res. 2019;11:156–69. https://doi.org/10.4168/aair.2019.11.2.156.
Liu X, Ng CL, Wang Y. The efficacy of sublingual immunotherapy for allergic diseases in Asia. Allergol Int. 2018;67:309–19. https://doi.org/10.1016/j.alit.2018.02.007.
Pawankar R, Mori S, Ozu C, Kimura S. Overview on the pathomechanisms of allergic rhinitis. Asia Pac Allergy. 2011;1:157–67. https://doi.org/10.5415/apallergy.2011.1.3.157.
Miyara M, Sakaguchi S. Natural regulatory T cells: mechanisms of suppression. Trends Mol Med. 2007;13:108–16. https://doi.org/10.1016/j.molmed.2007.01.003.
Presser K, Schwinge D, Wegmann M, Huber S, Schmitt S, Quaas A, Maxeiner JH, Finotto S, Lohse AW, Blessing M, Schramm C. Coexpression of TGF-beta1 and IL-10 enables regulatory T cells to completely suppress airway hyperreactivity. J Immunol. 2008;181:7751–8. https://doi.org/10.4049/jimmunol.181.11.7751.
Wilson DR, Lima MT, Durham SR. Sublingual immunotherapy for allergic rhinitis: systematic review and meta-analysis. Allergy. 2005;60:4–12. https://doi.org/10.1111/j.1398-9995.2005.00699.x.
Li B, Zhang X, Sun Z, Xu B, Wu J, Liu H, Han H, Wang L, Wu W. A novel strategy for the treatment of allergic rhinitis: regulating Treg/Th17 and Th1/Th2 balance in vivo by vitamin D. Comput Math Methods Med. 2022;2022:9249627. https://doi.org/10.1155/2022/9249627.
Joint Task Force on Practice P, American Academy of Allergy A, Immunology, American College of Allergy A, Immunology, Joint Council of Allergy A, Immunology. Allergen immunotherapy: a practice parameter second update. J Allergy Clin Immunol. 2007;120:S25–85. https://doi.org/10.1016/j.jaci.2007.06.019.
Savolainen J, Jacobsen L, Valovirta E. Sublingual immunotherapy in children modulates allergen-induced in vitro expression of cytokine mRNA in PBMC. Allergy. 2006;61:1184–90. https://doi.org/10.1111/j.1398-9995.2006.01206.x.
Romani N, Clausen BE, Stoitzner P. Langerhans cells and more: langerin-expressing dendritic cell subsets in the skin. Immunol Rev. 2010;234:120–41. https://doi.org/10.1111/j.0105-2896.2009.00886.x.
Valladeau J, Ravel O, Dezutter-Dambuyant C, Moore K, Kleijmeer M, Liu Y, Duvert-Frances V, Vincent C, Schmitt D, Davoust J, Caux C, Lebecque S, Saeland S. Langerin, a novel C-type lectin specific to Langerhans cells, is an endocytic receptor that induces the formation of Birbeck granules. Immunity. 2000;12:71–81. https://doi.org/10.1016/s1074-7613(00)80160-0.
Bykovskaia SN, Shurin GV, Graner S, Bunker ML, Olson W, Thomas R, Shurin MR, Marks S, Storkus WJ, Shogan J. Differentiation of immunostimulatory stem-cell- and monocyte-derived dendritic cells involves maturation of intracellular compartments responsible for antigen presentation and secretion. Stem Cells. 2002;20:380–93. https://doi.org/10.1634/stemcells.20-5-380.
Scadding GW, Shamji MH, Jacobson MR, Lee DI, Wilson D, Lima MT, Pitkin L, Pilette C, Nouri-Aria K, Durham SR. Sublingual grass pollen immunotherapy is associated with increases in sublingual Foxp3-expressing cells and elevated allergen-specific immunoglobulin G4, immunoglobulin A and serum inhibitory activity for immunoglobulin E-facilitated allergen binding to B cells. Clin Exp Allergy. 2010;40:598–606. https://doi.org/10.1111/j.1365-2222.2010.03462.x.
Allam JP, Niederhagen B, Bucheler M, Appel T, Betten H, Bieber T, Berge S, Novak N. Comparative analysis of nasal and oral mucosa dendritic cells. Allergy. 2006;61:166–72. https://doi.org/10.1111/j.1398-9995.2005.00965.x.
Iellem A, Mariani M, Lang R, Recalde H, Panina-Bordignon P, Sinigaglia F, D’Ambrosio D. Unique chemotactic response profile and specific expression of chemokine receptors CCR4 and CCR8 by CD4(+)CD25(+) regulatory T cells. J Exp Med. 2001;194:847–53. https://doi.org/10.1084/jem.194.6.847.
Authorship Contributions
Xinxin Zhang, Geping Wu, Xingkai Ma, and Lei Cheng participated in acquisition/collection of data and analysis or interpretation of data. Xinxin Zhang, Geping Wu, Xingkai Ma, and Lei Cheng participated in analysis or interpretation of data. Xinxin Zhang, Geping Wu, Xingkai Ma, and Lei Cheng participated in drafting the publication and/or revising it critically for important intellectual content and approved the final version for submission.
Authorship
All named authors meet the ICMJE criteria for authorship for this article, take responsibility for the integrity of the work as a whole, and have given their approval for this version to be published.
Funding
This study was supported by the Key Discipline Construction Project of Suzhou City, Project Number: SZXK202119. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript. The journal’s Rapid Service Fee was purchased using funding provided by the study sponsor.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
Xinxin Zhang, Geping Wu, Xingkai Ma, and Lei Cheng declare that they have no conflict of interest.
Compliance with ethics guidelines
The study was performed in accordance with relevant guidelines and regulations, following the approval of the licensing committee of Zhangjiagang Hospital affiliated to Soochow University, Suzhou, China (IRB#: ZJG7714; date of approval: 13 September 2018), and conformed to the tenets of the Declaration of Helsinki. Written informed consent was obtained from all participants. We thank the participants of the study.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Zhang, X., Wu, G., Ma, X. et al. Immune Cell Alterations and PI3K-PKB Pathway Suppression in Patients with Allergic Rhinitis Undergoing Sublingual Immunotherapy. Adv Ther 41, 777–791 (2024). https://doi.org/10.1007/s12325-023-02747-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12325-023-02747-z