Abstract
Introduction
US claims-based analyses emphasize the substantial hospitalization burden of patients with pulmonary arterial hypertension (PAH) and the significant need for improved monitoring and more timely interventions. A claims-based predictive model may be useful to assist healthcare providers and payers in identifying patients with PAH at increased hospitalization risk. To address this aim, we constructed statistical models using baseline patient variables available in administrative healthcare claims to predict patients’ risk for all-cause and PH-related hospitalization within 1 year of initiating ≥ 1 PAH indicated medication.
Methods
Adult patients with PAH who newly initiated ≥ 1 PAH indicated medication were selected from the MarketScan Commercial and Medicare Supplemental databases (January 1, 2009–January 31, 2019). Cox regression models were built with a randomly selected training set and evaluated using a validation set of remaining patients. Predictive variables for the models were selected in three steps: clinical knowledge, univariate analysis, and backward stepwise selection.
Results
Within 1 year of initiating ≥ 1 PAH indicated medication, 1502/3872 (38.8%) had an all-cause hospitalization and 950/3872 (24.5%) had a pulmonary hypertension (PH)-related hospitalization. Predictive risk factors for all-cause hospitalization were Quan–Charlson Comorbidity Index (CCI) score 2–3 [hazard ratio (HR) 1.229; P = 0.038] and ≥ 4 (HR 1.531; P < 0.001), claims-based frailty index (CFI) score > 1 (highest frailty level; HR 1.301; P = 0.018), hemoptysis (HR 1.254; P = 0.016), malaise/fatigue (HR 1.150; P = 0.037), history of PH-related hospitalization (HR 1.171; P = 0.011), non-PH-related ER visit (HR 1.713; P = 0.014), and higher non-PH-related outpatient visit cost (HR 1.069; P < 0.001). Predictive risk factors for PH-related hospitalization were female sex (HR 1.264; P = 0.004), Quan-CCI score ≥ 4 (HR 1.408; P = 0.008), portal hypertension (HR 1.565; P = 0.019), CFI score > 1 (HR 1.522; P = 0.002), dyspnea (HR 1.259; P = 0.023), and history of PH-related hospitalization (HR 1.273; P = 0.002).
Conclusions
The US claims-based predictive models showed acceptable performance to predict 1-year hospitalization among patients with PAH.
Similar content being viewed by others
Why carry out this study? |
US claims-based analyses emphasize the substantial hospitalization burden of patients with PAH and the significant need for improved monitoring and more timely interventions. |
A claims-based predictive model may be useful to assist healthcare providers and payers in identifying patients with PAH at increased hospitalization risk; therefore, we constructed statistical models using baseline patient variables available in administrative healthcare claims to predict patients’ risk for all-cause and PH-related hospitalization within 1 year of initiating at least one PAH indicated medication. |
What was learned from this study? |
The predictive models constructed from patients’ baseline characteristics and healthcare resource utilization and costs showed acceptable performance for predicting patients with PAH at high risk for all-cause and PH-related hospitalization. |
Such an administrative healthcare claims-based predictive tool may have utility for facilitating more expeditious care of this vulnerable patient population and may also help to avoid lengthy and costly hospitalizations. |
Furthermore, healthcare providers, payers, and other stakeholders may want to utilize the administrative healthcare claims-based assessment models to determine the needs of populations of patients with PAH in terms of health plan capacity, quality of care, monitoring, hospitalization burden, and cost estimates. |
Introduction
Pulmonary arterial hypertension (PAH) is a rare disease characterized by progressive pulmonary vascular resistance, eventual right heart failure, and high mortality [1]. It is frequently associated with hospitalization, an event indicative of disease progression and decreased survival [2,3,4]. A study using the Registry to Evaluate Early and Long-term PAH Disease Management (REVEAL Registry) reported that nearly 60% of patients newly diagnosed with PAH (N = 862) were hospitalized over a 3-year time period, with over 50% of the hospitalizations being PAH-related [3].
Hospitalizations contribute substantially not only to the clinical burden associated with PAH but also to its healthcare economic burden [2, 4,5,6]. A US claims-based study of 4009 patients with PAH reported that 57% had at least one PH-related hospitalization over a 35-month follow-up period; inpatient costs averaged $46,118 and $16,319 (2011 USD) among those commercially insured and those covered with Medicare Advantage plans, respectively [4]. Another US claims-based study of 3908 patients with PAH or a PAH-related condition reported a decline in the frequency of hospitalizations, all-cause and PH-related, after patients initiated a PAH indicated medication [i.e., 25% initiated endothelin receptor agonists (ERAs), 78% phosphodiesterase-5 inhibitors (PDE-5Is), and 0.7% soluble guanylate cyclase (sGC) stimulators; 95% initiated monotherapy] compared to during the 6 months prior to medication initiation [6]. Correspondingly, across the patient population, hospitalization costs declined from $32,322 to $18,531 per patient (2014 USD) [6].
The findings of these US claims-based analyses emphasize the substantial hospitalization burden of patients with PAH and the significant need for improved monitoring and more timely interventions. A claims-based predictive model may be useful to assist healthcare providers and payers in identifying patients with PAH at increased hospitalization risk. To address this aim, we constructed statistical models using baseline patient variables available in administrative healthcare claims to predict patients’ risk for all-cause and PH-related hospitalization within 1 year of initiating at least one PAH indicated medication.
Methods
Study Design, Data Sources, and Ethics Compliance
This study was a retrospective, observational, US claims-based study that utilized the IBM® MarketScan® Commercial Claims and Encounters (CCAE) and Medicare Supplemental databases, which contain only deidentified patient data and are fully compliant with the Health Insurance Portability and Accountability Act (HIPAA). As such, this study did not require ethics committee approval. The study was conducted in accordance with the Helsinki Declaration of 1964, and its later amendments.
Study Population
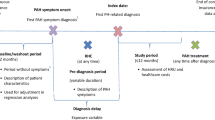
Patients 18 years of age or older who newly initiated at least one PAH indicated medication (ERAs, PDE-5Is, prostacyclin pathway agents, sGC stimulators) between January 1, 2009 and January 31, 2019 were selected from the MarketScan Commercial and Medicare Supplemental databases. The index date of a patient was 30 days after the date of the first claim for a PAH indicated medication. This 30-day window was utilized to determine initial treatment patterns, such as initiation of a second PAH indicated medication (treatment determination window). A 30-day grace period was chosen to allow time to complete clinical testing and administrative processes (i.e., prior authorization and Risk Evaluation and Mitigation Strategy) before adding another PAH therapy. The grace period is needed to avoid immortal time bias. This is important when the timing of PAH treatment initiation is likely to affect the hospitalization outcome. Patients were required to have had at least one documented diagnosis of PH (ICD-9-CM: 416.0, 416.8; ICD-10-CM: I27.0, I27.20, I27.21, I27.89) in an inpatient or emergency room (ER) setting or at least two documented diagnoses of PH that were 30 days or more apart in outpatient settings during a 12-month baseline period prior to the index date or during the 30-day treatment determination window. Patients were additionally required to be continuously enrolled with medical and pharmacy benefits during the baseline period and during the 30-day treatment determination window. Patients were excluded from the study population if they had a claim for any PAH indicated medication prior to 2009 or during the 12-month baseline period or if they were hospitalized during the treatment determination window. Also, patients diagnosed with chronic thromboembolic pulmonary hypertension or who had a pulmonary endarterectomy during the baseline period or treatment determination window were excluded.
Demographic and Clinical Characteristics
For each patient who met the study inclusion criteria, age, sex, US geographic region of residence, insurance type, health plan type, Quan–Charlson Comorbidity Index (Quan-CCI) score, comorbidities (N = 31), claims-based frailty index (CFI) score [7], PAH indicated medication class and therapy type (e.g., monotherapy, dual therapy, etc.), PAH-related symptoms/signs, and PH-related procedures were evaluated during the 12-month baseline period. Baseline usage of concomitant medications (N = 16) was additionally examined.
Baseline Non-PH- and PH-Related Healthcare Resource Utilization
Non-PH- and PH-related healthcare resource utilization (inpatient, ER, and outpatient visits) and associated costs were also evaluated during the baseline period. A medical service claim was considered PH-related based on a PH diagnosis at any position for inpatient or outpatient medical services. Otherwise, the encounter was considered non-PH-related.
Outcomes and Statistical Analyses
Patients were followed until the earliest event of hospitalization, health plan disenrollment, end of study, or end of the 1-year follow-up. The primary outcome of this analysis was time to all-cause hospitalization and the secondary outcome was time to PH-related hospitalization, both within 1 year from patients’ index dates. A PH-related hospitalization was defined as one with a PH diagnosis code at any position on the inpatient stay claim.
Survival models were constructed to evaluate the associations between time to all-cause and PH-related hospitalizations and potential predictive risk factors. Cox regression models were built with a training set randomly selected from the cohort and evaluated using a validation set of the remaining patients (ratio 0.8:0.2). Variables for the models were selected from the 86 evaluated baseline patient characteristics and healthcare resource utilization and cost measurements (Tables 1, 2, and 3) in three steps: (1) clinical knowledge covariate selection, (2) univariate analysis, and (3) backward stepwise selection (Fig. 1). In the univariate analysis, the chi-squared test based on simple Cox regression, log-rank test, and likelihood ratio test were applied to calculate the associations between continuous variables, binary variables, and multilevel categorical variables, respectively, with the outcome variables. Variables with a P value < 0.05 were retained. During the training process, the median of the predicted risk score of patients in the training set was chosen as the cutoff value between high- and low-risk patient groups for each model, which was then used to determine the high- and low-risk patient groups in the validation set. During the validation process, survival analyses of free from all-cause and PH-related hospitalization were conducted using Kaplan–Meier analysis with hazard ratios (HRs) and P values reported. The predictive accuracy of the final Cox regression models was measured with the concordance statistic (c-statistic). The survival package in R was used to perform the log-rank test and Cox regression. The MASS package in R was used to perform stepwise feature selection and the lifeline package in Python was used to plot the survival curves.
Other Statistical Analyses
Descriptive statistics were utilized to summarize the measured patient demographic and clinical characteristics and healthcare resource utilization and the associated costs and were carried out in RStudio 1.4.1717 (Boston, MA).
Results
Study Population
The final study cohort included 3872 patients who newly initiated at least one PAH indicated medication. The evaluated baseline demographic and clinical characteristics are shown in Table 1 and Supplementary Material Table 1, respectively. The mean age of the study cohort was 63.1 years, 60.0% were female, 56.6% had commercial insurance, and approximately one-half (49.1%) had preferred provider organization health plans. Mean Quan-CCI score was 3.2 and the most prevalent comorbidities were hypertension (78.3%), congestive heart failure (55.4%), hyperlipidemia (50.4%), coronary artery disease (46.0%), and chronic obstructive pulmonary disorder (43.7%). Mean CFI score was 0.6 and 52.5% of the patients with PAH had a score between 0.2 and 1.
A majority (61.2%) of the study cohort initiated a PDE-5I and 95.7% were on monotherapy. The most common PAH-related symptoms/signs were dyspnea (78.6%), chest pain (44.7%), cardiomegaly (29.9%), peripheral edema (26.1%), and malaise/fatigue (24.0%).
Baseline Non-PH- and PH-Related Healthcare Resource Utilization
During the 12-month baseline period, the mean number of non-PH-related hospitalizations was 0.7 per patient; non-PH-related ER and outpatient visits averaged 0.5 and 30.4, respectively, per patient (Supplementary Material Table 2). The mean number of PH-related hospitalizations was 0.6 per patient; PH-related ER and outpatient visits averaged 0.0 and 3.8, respectively, per patient (Supplementary Material Table 2).
All-Cause and PH-Related Hospitalizations
Among the study cohort, 1502 (38.8%) had an all-cause hospitalization and 950 (24.5%) had a PH-related hospitalization within 1 year of initiating at least one PAH indicated medication.
Predictive Risk Factors of All-Cause and PH-Related Hospitalizations
Of the 86 evaluated baseline patient characteristics and healthcare resource utilization and cost measurements, 25 were selected for all-cause hospitalization by univariate analysis of the training set and 16 for PH-related hospitalization. Subsequent backward stepwise selection and Cox regression model training identified eight independent predictive risk factors for all-cause hospitalization and six for PH-related hospitalization within 1 year of initiating at least one PAH indicated medication. Kaplan–Meier survival curves (free of all-cause and PH-related hospitalization) of the high- and low-risk groups in the validation set predicted by each model are plotted in Fig. 2. HRs were 2.39 and 1.95 for the patient group at high risk for all-cause hospitalization and PH-related hospitalization, respectively. The final risk prediction models had a c-statistic of 0.64 for all-cause hospitalization and 0.61 for PH-related hospitalization in the validation sample.
The eight baseline selected predictive risk factors for all-cause hospitalization were Quan-CCI score 2–3 (HR 1.229; P = 0.038) and ≥ 4 (HR 1.531; P < 0.001), CFI score > 1 (HR 1.301; P = 0.018), hemoptysis (HR 1.254; P = 0.016), malaise/fatigue (HR 1.150; P = 0.037), history of PH-related hospitalization (HR 1.171; P = 0.011), non-PH-related ER visit (HR 1.713; P = 0.014), and higher non-PH-related outpatient visit cost (HR 1.069; P < 0.001) (Table 2).
The six baseline selected predictive risk factors for PH-related hospitalization were female sex (HR 1.264; P = 0.004), Quan-CCI score ≥ 4 (HR 1.408; P = 0.008), portal hypertension (HR 1.565; P = 0.019), CFI score > 1 (HR 1.522; P = 0.002), dyspnea (HR 1.259; P = 0.023), and history of PH-related hospitalization (HR 1.273; P = 0.002) (Table 3).
Discussion
The aim of this study was to construct Cox regression models using baseline patient variables available in administrative healthcare claims to predict patients’ risk for hospitalization within 1 year of initiating at least one PAH indicated medication. The final models showed acceptable performance for predicting those patients at high risk for all-cause and PH-related hospitalization. In the population of 3872 patients diagnosed with PH who newly initiated at least one PAH indicated medication, eight baseline selected predictive risk factors of having an all-cause hospitalization within 1 year were identified and included higher general comorbidity level according to Quan-CCI score, highest frailty level, symptoms of hemoptysis and malaise/fatigue, history of PH-related hospitalization, history of non-PH-related ER visit, and higher non-PH-related outpatient visit cost. The six baseline selected predictive risk factors of having a PH-related hospitalization within 1 year were female sex, higher general comorbidity level, portal hypertension, highest frailty level, dyspnea, and history of PH-related hospitalization.
The predictive models for distinguishing patients with PAH at higher risk for hospitalization may have utility for facilitating more expeditious care of this vulnerable patient population and may also help to avoid lengthy and costly hospitalizations. Currently, there are several algorithms for survival/prognosis assessment of patients with PAH derived from data collected from patient registries and/or published guidelines, including the REVEAL risk equation/score [8,9,10], the French Pulmonary Hypertension Network (FPHN) registry risk equation [11], the Scottish composite score [12], and the 2015 European Society of Cardiology (ESC) and the European Respiratory Society (ERS) PH guidelines risk table [13]. These risk algorithms utilize multiple patient parameters, such as 6-min walking distance, World Health Organization Functional Class, hemodynamic measurements, and other clinically measured variables, and are helpful for healthcare providers to assess patient prognosis and survival and to make treatment decisions. Most of the patient functional and clinical characteristics used to assess PAH patient prognosis/survival in these equations/scores are not comprehensively available in administrative healthcare claims data. It was not until the risk calculator REVEAL 2.0 [10] that all-cause hospitalization was included in the risk assessment of patients with PAH. Since healthcare providers, payers, and other stakeholders may want to assess hospitalization risk to determine the needs of individual patients in addition to populations of patients with PAH in terms of health plan capacity, quality of care, monitoring, hospitalization burden, and cost estimates, a simpler administrative healthcare claims-based assessment of hospitalization risk of patients with PAH may be useful. Such a tool may also prove useful as an adjunct to the validated clinical risk assessments and evidence-based guidelines for improving access to care and to maintain a reduced hospitalization burden among patients with PAH.
Hospitalization as an outcome of patients with PAH is a marker of disease progression, rehospitalization, and reduced survival [2,3,4, 14,15,16]. In a post hoc analysis of the phase III GRIPHON trial, patients who had hospitalization for worsening of PAH were significantly more likely to die during the 1-year follow-up period than those who did not (HR for mortality within 3 months 6.55; 95% confidence interval 4.02–10.67; HR for mortality within 12 months 3.25; 95% confidence interval 1.94–5.44) [14, 15]. In a real-world observational analysis of 3001 patients with PAH in the REVEAL Registry, all-cause hospitalization was also highly predictive of mortality in 1 year [16]. In our current study, 39% and 25% of the patient population had an all-cause and PH-related hospitalization, respectively, within 1 year of initiating at least one PAH indicated medication. With such high rates of hospitalization among newly treated patients with PAH and the significant association of hospitalization with proximate mortality, the event should trigger modifications in the care pathways of patients with PAH. In fact, in both of our predictive models of all-cause and PH-related hospitalization risk, prior PH-related hospitalizations were a significant predictor of hospitalization within a year of initiating at least one PAH indicated medication. Thus, earlier intervention, including treatment escalation for PAH, as well as management of comorbidity, which was highly prevalent among the study population (41% with Quan-CCI score of ≥ 4 and 38% with a score of 2–3) and also a significant predictive factor in both our models, among patients with PAH at high risk for hospitalization prior to the event will likely be helpful to avoid the possible consequences of hospitalization. Early treatment interventions among patients with PAH, a very rapid progressive disease, has substantial clinical evidence for improving patient outcomes [17].
Meta-analyses including a multitude of clinical trials of PAH indicated medications have reported up to an approximately 40% risk reduction in clinical worsening (an endpoint including but not limited to hospitalization and death) for combination therapy, the utilization of two or more PAH indicated medications that target multiple pathways of disease pathogenesis, versus monotherapy [18, 19]. On the basis of such favorable clinical trial evidence for combination therapy, it is recommended in the ESC/ERS PH treatment guidelines initially for treatment-naïve newly diagnosed patients with PAH and those who are already treated [13, 20]. Despite the evidence-based 2015 treatment recommendations, the utilization of combination therapy among patients with PAH in the USA has had a slow uptake [21], reaching a reported maximum of approximately 20% in an observational study of 3116 patients with PAH selected from a US administrative commercial claims database between January 2014 and June 2019 [22]. Furthermore, in this study by Ogbomo et al. [22] of hospitalized patients with PAH, 65% were treated with monotherapy prior to hospitalization and less than 7% had treatment escalation following an all-cause hospitalization. These study findings indicate that in the USA, routine treatment practices of patients with PAH do not involve treatment escalation with combination therapy to a great extent even following a hospitalization event.
Since combination therapy is costly [23], it is important for healthcare providers and payers to have practical and easy-to-use risk assessment tools to predict the need for possible treatment interventions among patients with PAH. Alongside those of other recent real-world studies [21, 22, 24], the findings of the current study regarding the high proportions of patients with PAH who experienced hospitalization within 1 year following initiation of at least one PAH indicated medication, with 96% treated with monotherapy from 2009 through 2019, suggest that there remain significant barriers to utilization of combination therapy even among high-risk patients. In a study that examined the transition of care of patients with PAH from hospitalization to the outpatient setting and its improvement describe prior authorization requirements, PAH medication complexity, required enrollment into risk evaluation programs, and specialty pharmacy dispensing as barriers to receiving treatment with PAH indicated medications [25].
Limitations
The findings of this US claims-based analysis should be interpreted in the context of certain limitations. Firstly, this study was observational and the influence of patient characteristics and prior healthcare resource utilization on hospitalization outcomes is limited to associations and cannot be interpreted as causal. Administrative healthcare claims are not created for research purposes but are collected for reimbursement purposes and they may contain potential coding errors and inconsistences. Importantly, the presence of a pharmacy claim does not necessarily indicate that the medication was taken as prescribed or if treatment began at the date of the claim. Also, because of data source limitations, medications prescribed in the inpatient setting are not captured, and neither are those supplied as samples or purchased over-the counter. The MarketScan data sources are comprised of patient claims distributed disproportionately across the USA and the observational data collected herein may not generalize to the entire or particular US populations of patients with PAH , such as those who receive care at a Pulmonary Hypertension Association accredited center or those covered by other insurance types not represented in the data sources. Furthermore, there are no specific ICD-9/10 codes that identify PAH; hence, we required that patients included in this study had newly initiated at least one PAH indicated medication (ERAs, PDE-5Is, prostacyclin pathway agents, sGC stimulators) to improve the identification of solely patients with PAH. However, it is possible that a few patients included in this study may have another form of PH and were prescribed a PAH indicated medication by their healthcare provider.
In this study, patients hospitalized within 30 days after their first claim for a PAH medication (i.e., treatment determination window) were not included in the analysis. The predictive risk factors for hospitalization among newly treated patients with PAH identified in this study using administrative healthcare claims should not be used in place of physicians’ assessments and clinical/laboratory tests, but potentially as an adjunct to such evaluations for determining patient hospitalization risk and to examine PAH patient care in healthcare systems. Additionally, it should not be inferred that patients in the low-risk hospitalization group do not require modifications in their treatment path to prevent poor outcomes. This study was conducted on a patient population identified prior to the onset of the coronavirus disease 2019 (COVID-19) pandemic and the symptomatology and long-term sequalae of COVID-19, especially that related to the cardiopulmonary vasculature, as well as the barriers to treatment that occurred during the first part of the pandemic, which may predispose some patients with PAH to worse outcomes [26,27,28].
Conclusions
The predictive models constructed from patients’ baseline characteristics and healthcare resource utilization and costs showed acceptable performance for predicting patients with PAH at high risk for all-cause and PH-related hospitalization. Such an administrative healthcare claims-based predictive tool could be used to facilitate monitoring and timely escalation of treatment in higher-risk patients, potentially improving patients’ clinical and economic outcomes.
References
Badlam JB, Bull TM. Steps forward in the treatment of pulmonary arterial hypertension: latest developments and clinical opportunities. Ther Adv Chronic Dis. 2017;8(2–3):47–64.
Bergot E, De Leotoing L, Bendjenana H, et al. Hospital burden of pulmonary arterial hypertension in France. PLoS ONE. 2019;14(9): e0221211.
Burger CD, Long PK, Shah MR, et al. Characterization of first-time hospitalizations in patients with newly diagnosed pulmonary arterial hypertension in the REVEAL registry. Chest. 2014;146(5):1263–73.
Burke JP, Hunsche E, Régulier E, Nagao M, Buzinec P, Drake W III. Characterizing pulmonary hypertension-related hospitalization costs among Medicare Advantage or commercially insured patients with pulmonary arterial hypertension: a retrospective database study. Am J Manag Care. 2015;21(3 Suppl):s47-58.
Kirson NY, Birnbaum GH, Ivanova JI, Waldman T, Joish V, Williamson T. Excess costs associated with patients with pulmonary arterial hypertension in a US privately insured population. Appl Health Econ Health Policy. 2011;9(5):293–303.
Burger CD, Ozbay AB, Lazarus HM, et al. Treatment patterns and associated health care costs before and after treatment initiation among pulmonary arterial hypertension patients in the United States. J Manag Care Spec Pharm. 2018;24(8):834–42.
Segal JB, Chang H-Y, Du Y, Walston JD, Carlson MC, Varadhan R. Development of a claims-based frailty indicator anchored to a well-established frailty phenotype. Med Care. 2017;55(7):716–22.
Benza RL, Miller DP, Gomberg-Maitland M, et al. Predicting survival in pulmonary arterial hypertension: insights from the registry to evaluate early and long-term pulmonary arterial hypertension disease management (REVEAL). Circulation. 2010;122(2):164–72.
Benza RL, Gomberg-Maitland M, Miller DP, et al. The REVEAL Registry risk score calculator in patients newly diagnosed with pulmonary arterial hypertension. Chest. 2012;141(2):354–62.
Benza RL, Gomberg-Maitland M, Elliott CG, et al. Predicting survival in patients with pulmonary arterial hypertension: the REVEAL risk score calculator 2.0 and comparison with ESC/ERS-based risk assessment strategies. Chest. 2019;156(2):323–37.
Humbert M, Sitbon O, Yaici A, et al. Survival in incident and prevalent cohorts of patients with pulmonary arterial hypertension. Eur Respir J. 2010;36(3):549–55.
Lee W-TN, Ling Y, Sheares KK, Pepke-Zaba J, Peacock AJ, Johnson MK. Predicting survival in pulmonary arterial hypertension in the UK. Eur Respir J. 2012;40(3):604–11.
Galié N, Humbert M, Vachiery JL, et al. 2015 ESC/ERS Guidelines for the diagnosis and treatment of pulmonary hypertension: The Joint Task Force for the Diagnosis and Treatment of Pulmonary Hypertension of the European Society of Cardiology (ESC) and the European Respiratory Society (ERS): Endorsed by: Association for European Paediatric and Congenital Cardiology (AEPC), International Society for Heart and Lung Transplantation (ISHLT). Eur Respir J. 2015;46(4):903–75.
Sitbon O, Channick R, Chin KM, et al. Selexipag for the treatment of pulmonary arterial hypertension. N Engl J Med. 2015;373(26):2522–33.
McLaughlin VV, Hoeper MM, Channick RN, et al. Pulmonary arterial hypertension-related morbidity is prognostic for mortality. J Am Coll Cardiol. 2018;71(7):752–63.
Frost AE, Badesch DB, Miller DP, Benza RL, Meltzer LA, McGoon MD. Evaluation of the predictive value of a clinical worsening definition using 2-year outcomes in patients with pulmonary arterial hypertension: a REVEAL registry analysis. Chest. 2013;144(5):1521–9.
Humbert M, Coghlan JG, Khanna D. Early detection and management of pulmonary arterial hypertension. Eur Respir Rev. 2012;21(126):306–12.
Lajoie AC, Lauzière G, Lega J-C, et al. Combination therapy versus monotherapy for pulmonary arterial hypertension: a meta-analysis. Lancet Respir Med. 2016;4(4):291–305.
Fox BD, Shtraichman O, Langleben D, Shimony A, Kramer MR. Combination therapy for pulmonary arterial hypertension: a systematic review and meta-analysis. Can J Cardiol. 2016;32(12):1520–30.
Galiè N, Channick RN, Frantz RP, et al. Risk stratification and medical therapy of pulmonary arterial hypertension. Eur Respir J. 2019;53(1):1801889.
Studer S, Hull M, Pruett J, Elliot C, Tsang Y, Drake W III. Retrospective database analysis of treatment patterns among patients with pulmonary arterial hypertension. Pulm Ther. 2020;6(1):79–92.
Ogbomo A, Tsang Y, Kariburyo F, Tsai W-L, Panjabi S. Real-world analysis of treatment patterns among hospitalized patients with pulmonary arterial hypertension. Pulm Ther. 2021;7(2):575–90.
Studer S, Hull M, Pruett KE, Tsang Y, Drake III W. Treatment patterns, healthcare resource utilization, and healthcare costs among patients with pulmonary arterial hypertension in a real-world US database. Pulm Circ. 2019;9(1):2045894018816294.
Gauthier-Loiselle M, Tsang Y, Lefebvre P, et al. Development and evaluation of a predictive algorithm for unsatisfactory response among patients with pulmonary arterial hypertension using health insurance claims data. Curr Med Res Opin. 2022;38(6):1019–30.
Martirosov AL, Smith ZR, Hencken L, et al. Improving transitions of care for critically ill adult patients on pulmonary arterial hypertension medications. Am J Health Syst Pharm. 2020;77(12):958–65.
Lee JD, Burger CD, Delossantos GB, et al. A survey-based estimate of COVID-19 incidence and outcomes among patients with pulmonary arterial hypertension or chronic thromboembolic pulmonary hypertension and impact on the process of care. Ann Am Thorac Soc. 2020;17(12):1576–82.
Farmakis IT, Karyofyllis P, Frantzeskaki F, et al. Incidence and outcomes of COVID-19 in patients with pulmonary arterial hypertension and chronic thromboembolic pulmonary hypertension: Data from the Hellenic pulmOnary hyPertension rEgistry (HOPE). Hellenic J Cardiol. 2022;64:93–6.
Cascino TM, Desai AA, Kanthi Y. At a crossroads: coronavirus disease 2019 recovery and the risk of pulmonary vascular disease. Curr Opin Pulm Med. 2021;27(5):342–9.
Acknowledgements
Funding
This study, preparation of this manuscript, and rapid service fee were sponsored and funded by Janssen Business Technology Commercial Data Sciences and Janssen Scientific Affairs, LLC.
Medical Writing/Editorial Assistance
Medical writing and editorial assistance for preparation of this manuscript was provided by Jay Lin and Melissa Lingohr-Smith of Novosys Health and was funded by Janssen, LLC.
Authorship
All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this article, take responsibility for the integrity of the work as a whole, and have given their approval for this version to be published.
Author Contributions
All authors, Chang Zhang, Yuen Tsang, Jinghua He, and Sumeet Panjabi contributed to the study conception and design, data collection and analysis, drafting and revising of the manuscript, and approved the final manuscript version.
Disclosures
Chang Zhang, Yuen Tsang, Jinghua He, and Sumeet Panjabi are employees of Janssen, LLC and may have stock ownership.
Compliance with Ethics Guidelines
This study was a retrospective, observational, US claims-based study that utilized the IBM® MarketScan® Commercial Claims and Encounters (CCAE) and Medicare Supplemental databases, which contain only deidentified patient data and are fully compliant with the Health Insurance Portability and Accountability Act (HIPAA). As such, this study did not require ethics committee approval. The study was conducted in accordance with the Helsinki Declaration of 1964, and its later amendments.
Data Availability
The data for this US claims-based analysis are contained within the article or are available upon request from the corresponding author.
Prior Publication
Some aspects of this study were previously presented at the Academy of Managed Care Pharmacy (AMCP) Annual Meeting, March 29–April 1, 2022, Chicago, IL USA.
Author information
Authors and Affiliations
Corresponding author
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Zhang, C., Tsang, Y., He, J. et al. Predicting Risk of 1-Year Hospitalization Among Patients with Pulmonary Arterial Hypertension. Adv Ther 40, 2481–2492 (2023). https://doi.org/10.1007/s12325-023-02501-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12325-023-02501-5