Abstract
Introduction
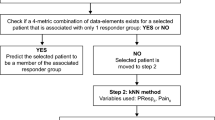
We sought to identify and characterize distinct responder profiles among osteoarthritis (OA) subjects treated with tanezumab, nonsteroidal anti-inflammatory drugs (NSAIDs), or placebo.
Methods
Subject-level data were derived from three randomized, double-blind, placebo- or NSAID-controlled trials of tanezumab in subjects with moderate-to-severe OA. Subjects received subcutaneous tanezumab (2.5 mg, n = 1527; 5 mg, n = 1279) every 8 weeks, oral NSAIDs (n = 994) daily, or placebo (n = 513). Group-based trajectory modeling (GBTM, an application of finite mixture statistical modeling that uses response trajectory to identify and summarize complex patterns in longitudinal data) was used to identify subgroups of subjects following similar patterns of response in each treatment arm, based on daily pain intensity scores from baseline through Week 16. We then examined whether subject-related variables were associated with any of the subgroups using multinomial logistic regression.
Results
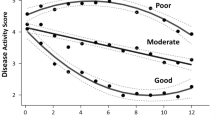
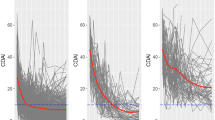
A three-subgroup/four-inflection point trajectory model was selected based on clinical and statistical considerations. The subgroups were high responders (substantial pain improvement and a large majority of members achieved ≥ 30% improvement before Week 16), medium responders (gradual pain improvement and a majority of members achieved ≥ 30% improvement by Week 16), and non-responders (little to no pain improvement over 16 weeks). Across all treatments, fluctuation in pain intensity in the week prior to treatment was consistently associated with treatment response. Other variables were positively (age, body mass index, days of rescue medication use) or negatively (severity of disease based on Kellgren-Lawrence grading) associated with response but effects were small and/or varied across treatments.
Conclusions
Across all treatments, GBTM identified three subgroups of subjects that were characterized by extent of treatment response (high, medium, and non-responders). Similar analyses (e.g., grouping of subjects based on response trajectory and identification of subgroup-related variables) in other studies of OA could inform clinical trial design and/or treatment approaches. (NCT02697773; NCT02709486; NCT02528188).

Similar content being viewed by others
References
Edwards RR, Dworkin RH, Turk DC, et al. Patient phenotyping in clinical trials of chronic pain treatments: IMMPACT recommendations. Pain. 2016;157:1851–71.
Schnitzer TJ, Easton R, Pang S, et al. Effect of tanezumab on joint pain, physical function, and patient global assessment of osteoarthritis among patients with osteoarthritis of the hip or knee: a randomized clinical trial. JAMA. 2019;322:37–48.
Berenbaum F, Blanco FJ, Guermazi A, et al. Subcutaneous tanezumab for osteoarthritis of the hip or knee: efficacy and safety results from a 24-week randomised phase III study with a 24-week follow-up period. Ann Rheum Dis. 2020;79:800–10.
Hochberg MC, Carrino JA, Schnitzer TJ, et al. Long-term safety and efficacy of subcutaneous tanezumab versus nonsteroidal antiinflammatory drugs for hip or knee osteoarthritis: a randomized trial. Arthritis Rheumatol. 2021;73:1167–77.
Nagin DS. Group-based trajectory modeling: an overview. Ann Nutr Metab. 2014;65:205–10.
Nagin DS, Odgers CL. Group-based trajectory modeling in clinical research. Annu Rev Clin Psychol. 2010;6:109–38.
Törmälehto S, Aarnio E, Mononen ME, Arokoski JPA, Korhonen RK, Martikainen JA. Eight-year trajectories of changes in health-related quality of life in knee osteoarthritis: Data from the Osteoarthritis Initiative (OAI). PLoS ONE. 2019;14: e0219902.
Johnson AJ, Vasilopoulos T, Booker SQ, et al. Knee pain trajectories over 18 months in non-Hispanic Black and non-Hispanic White adults with or at risk for knee osteoarthritis. BMC Musculoskelet Disord. 2021;22:415.
Song J, Lee J, Lee YC, et al. Sleep Disturbance Trajectories in Osteoarthritis. J Clin Rheumatol. 2021;27:e440–5.
Han A, Gellhorn AC. Trajectories of Quality of Life and Associated Risk Factors in Patients With Knee Osteoarthritis: Findings From the Osteoarthritis Initiative. Am J Phys Med Rehabil. 2018;97:620–7.
Dworkin RH, Turk DC, Wyrwich KW, et al. Interpreting the clinical importance of treatment outcomes in chronic pain clinical trials: IMMPACT recommendations. J Pain. 2008;9:105–21.
Jones BL, Nagin DS, Roeder K. A SAS Procedure Based on Mixture Models for Estimating Developmental Trajectories. Sociological Methods & Research. 2001;29:374–93.
Bonin Pinto C, Barroso J, Schinitzer TJ. Characterization and implications of daily pain variability and response to treatment in osteoarthritis clinical trials. Osteoarthritis Cartilage. 2021;29:S277.
Treister R, Honigman L, Lawal OD, Lanier RK, Katz NP. A deeper look at pain variability and its relationship with the placebo response: results from a randomized, double-blind, placebo-controlled clinical trial of naproxen in osteoarthritis of the knee. Pain. 2019;160:1522–8.
Schneider S, Junghaenel DU, Keefe FJ, Schwartz JE, Stone AA, Broderick JE. Individual differences in the day-to-day variability of pain, fatigue, and well-being in patients with rheumatic disease: associations with psychological variables. Pain. 2012;153:813–22.
Den Teuling NGP, Pauws SC, van den Heuvel ER. A comparison of methods for clustering longitudinal data with slowly changing trends. Commun Stat Simul Comput. 2021:1–28.
Pfizer Inc. Pfizer reports third-quarter 2021 results. 2021. https://investors.pfizer.com/investor-news/press-release-details/2021/PFIZER-REPORTS-THIRD-QUARTER-2021-RESULTS/default.aspx. Accessed 20 April 2022.
Eli Lilly and Company. Lilly reports robust third-quarter 2021 financial results as pipeline success strengthens future growth potential. 2021. https://investor.lilly.com/static-files/a0b77c52-a997-41c1-9534-5f465903a0b4. Accessed 20 April 2022.
Acknowledgements
Funding
This study was funded by Pfizer and Eli Lilly and Company. The journal’s Rapid Service fee was funded by Pfizer and Eli Lilly and Company.
Medical Writing/Editorial Assistance
Medical writing support was provided by Matt Soulsby, PhD, CMPP, of Engage Scientific Solutions and funded by Pfizer and Eli Lilly and Company.
Authorship
All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this article, take responsibility for the integrity of the work as a whole, and have given their approval for this version to be published.
Author Contributions
All authors contributed to the (1) conception/design of the study and interpretation of data, (2) drafting the article or revising it critically for important intellectual content, and (3) final approval of the version to be submitted. Gianluca Bonfanti and Roger Edwards were also involved in analysis of data.
Disclosures
Thomas J. Schnitzer reports clinical research study support from Pfizer, Lilly, Regeneron, Galapagos, Taiwan Liposome Corporation, and Anika Therapeutics and has served as a consultant or on an advisory board for Pfizer, Eli Lilly and Company, Glaxo-Smith Kline, AstraZeneca, Noven, Galapagos, and Merck. Gianluca Bonfanti is an employee of Engineering Ingegneria Informatica, a paid sub-contractor to Health Services Consulting Corporation in conjunction with this study and development of this manuscript. Joanna Atkinson is a full-time employee of Pfizer, LTD. Sean Donevan is a full-time employee of, and owns stock/options in, Pfizer Inc. Lars Viktrup is a full-time employee of, and owns stock/options in, Eli Lilly and Company. Joana Barroso has received research support from Grünenthal. Ed Whalen is a full-time employee of, and owns stock/options in, Pfizer Inc. Roger A. Edwards is an owner of Health Services Consulting Corporation, a paid consultant by Pfizer in connection with this study and development of the manuscript.
Compliance with Ethics Guidelines
The studies included in this analysis were approved by an institutional review board or independent ethics committee at each study center. All patients provided written informed consent before participating. The studies were conducted in compliance with the Declaration of Helsinki and all International Conference on Harmonization Good Clinical Practice guidelines. Please see the primary study publications for more detail.
Data Availability
Upon request, and subject to review, Pfizer will provide the data that support the findings of this study. Subject to certain criteria, conditions, and exceptions, Pfizer may also provide access to the related individual de-identified participant data. See https://www.pfizer.com/science/clinical-trials/trial-data-and-results for more information.
Author information
Authors and Affiliations
Corresponding author
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Schnitzer, T.J., Bonfanti, G., Atkinson, J. et al. Characterizing 16-Week Responder Profiles Using Group-Based Trajectory Modeling in Over 4300 Clinical Trial Participants Receiving Pharmaceutical Treatment for Moderate to Severe Osteoarthritis. Adv Ther 39, 4742–4756 (2022). https://doi.org/10.1007/s12325-022-02290-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12325-022-02290-3