Abstract
Introduction
Rheumatoid arthritis (RA) is a chronic and refractory autoimmune disease characterized by synovial inflammation with unknown aetiology. Immune system dysfunction mediated by CD4+ T lymphocytes, which is regulated by the cytokine osteopontin (OPN), plays an important role in the pathogenesis of RA.
Methods
In this study, the levels of peripheral CD4+ T subsets and serum OPN in patients with active RA were measured and analysed to determine the possible pathogenesis of RA and to provide potential therapeutic targets.
Results
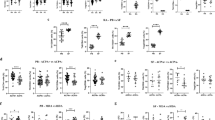
Serum OPN levels in both patients with active RA and patients with refractory RA were higher than those in healthy controls (HCs). Compared with HCs, the absolute numbers of Th2 cells increased in patients with active RA, while the absolute counts of Th1 and Treg cells decreased. There was no significant difference in CD4+ T subset levels between new-onset and refractory patients. As the condition persisted or deteriorated, a gradual increase in the levels of OPN and gradual declines in the absolute counts of Th1 and Treg cells were observed in patients with active RA. The fewest Th1 and Treg cells and the highest OPN levels were observed in patients with high disease activity. The serum OPN level was only significantly negatively correlated with the absolute counts of Treg cells in the CD4+ T lymphocyte subsets.
Conclusions
Fewer Treg cells with the increase in disease activity may be related to the increased OPN concentration, which may provide new ideas and directions for the targeted immunoregulatory treatment of RA.




Similar content being viewed by others
References
Wolfe F, Hawley DJ. The longterm outcomes of rheumatoid arthritis: Work disability: a prospective 18 year study of 823 patients. J Rheumatol. 1998;25(11):2108–17.
Woolf AD, Pfleger B. Burden of major musculoskeletal conditions. Bull World Health Organ. 2003;81(9):646–56.
Deane KD, Demoruelle MK, Kelmenson LB, et al. Genetic and environmental risk factors for rheumatoid arthritis. Best Pract Res Clin Rheumatol. 2017;31(1):3–18.
Zhang SX, Wang J, Wang CH, et al. Low-dose IL-2 therapy limits the reduction in absolute numbers of circulating regulatory T cells in rheumatoid arthritis. Ther Adv Musculoskelet Dis. 2021. https://doi.org/10.1177/1759720X211011370.
He J, Zhang R, Shao M, et al. Efficacy and safety of low-dose IL-2 in the treatment of systemic lupus erythematosus: a randomised, double-blind, placebo-controlled trial. Ann Rheum Dis. 2020;79(1):141–9.
Wang J, Zhang SX, Hao YF, et al. The numbers of peripheral regulatory T cells are reduced in patients with psoriatic arthritis and are restored by low-dose interleukin-2. Ther Adv Chronic Dis. 2020;11:2040622320916014.
Firestein GS. Evolving concepts of rheumatoid arthritis. Nature. 2003;423(6937):356–61.
Hamburg JPV, Corneth OBJ, Paulissen SMJ, et al. IL-17/Th17 mediated synovial inflammation is IL-22 independent. Ann Rheum Dis. 2013;72(10):1700.
Kawai K, Uchiyama M, Hester J, et al. Regulatory T cells for tolerance. Hum Immunol. 2018;79(5):294–303.
Ashkar S, Weber GF, Panoutsakopoulou V, et al. Eta-1 (osteopontin): an early component of type-1 (cell-mediated) immunity. Science. 2000;287(5454):860–4.
Diao H, Kon S, Iwabuchi K, et al. Osteopontin as a mediator of NKT cell function in T cell-mediated liver diseases. Immunity. 2004;21(4):539–50.
Diao H, Iwabuchi K, Li L, et al. Osteopontin regulates development and function of invariant natural killer T cells. Proc Natl Acad Sci USA. 2008;105(41):15884–9.
Chung JW, Kim MS, Piao ZH, et al. Osteopontin promotes the development of natural killer cells from hematopoietic stem cells. Stem Cells. 2008;26(8):2114–23.
Yumoto K, Ishijima M, Rittling SR, et al. Osteopontin deficiency protects joints against destruction in anti-type II collagen antibody-induced arthritis in mice. Proc Natl Acad Sci USA. 2002;99(7):4556–61.
Aletaha D, Neogi T, Silman AJ, et al. 2010 rheumatoid arthritis classification criteria: an American College of Rheumatology/European League Against Rheumatism collaborative initiative. Ann Rheum Dis. 2010;69(9):1580–8.
Arnett FC, Edworthy SM, Bloch DA, et al. The American Rheumatism Association 1987 revised criteria for the classification of rheumatoid arthritis. Arthritis Rheum. 1988;31(3):315–24.
Van der Linden MP, Knevel R, Huizinga TW, et al. Classification of rheumatoid arthritis comparison of the 1987 ACR and 2010 ACR/EULAR criteria. Arthritis Rheum. 2010. https://doi.org/10.1002/art.30100.
Petrow PK, Hummel KM, Schedel J, et al. Expression of osteopontin messenger RNA and protein in rheumatoid arthritis: effects of osteopontin on the release of collagenase 1 from articular chondrocytes and synovial fibroblasts. Arthritis Rheum. 2000;43(7):1597.
Sun PF, Kong WK, Liu L, et al. Osteopontin accelerates chondrocyte proliferation in osteoarthritis rats through the NF-kappab signaling pathway. Eur Rev Med Pharmacol Sci. 2020;24(6):2836–42.
Xu G, Nie H, Li N, et al. Role of osteopontin in amplification and perpetuation of rheumatoid synovitis. J Clin Invest. 2005;115(4):1060–7.
Yamamoto N, Sakai F, Kon S, et al. Essential role of the cryptic epitope SLAYGLR within osteopontin in a murine model of rheumatoid arthritis. J Clin Invest. 2003;112(2):181–8.
Miao M, Hao Z, Guo Y, et al. Short-term and low-dose IL-2 therapy restores the Th17/Treg balance in the peripheral blood of patients with primary Sjogren’s syndrome. Ann Rheum Dis. 2018;77(12):1838–40.
Wang J, Zhang SX, Hao YF, et al. The numbers of peripheral regulatory T cells are reduced in patients with psoriatic arthritis and are restored by low-dose interleukin-2. Ther Adv Chronic Dis. 2020;11:1–12.
Zhang SX, Wang J, Sun HH, et al. Circulating regulatory T cells were absolutely decreased in dermatomyositis/polymyositis patients and restored by low-dose IL-2. Ann Rheum Dis. 2019. https://doi.org/10.1136/annrheumdis-2019-216246.
Miyara M, Ito Y, Sakaguchi S. TREG-cell therapies for autoimmune rheumatic diseases. Nat Rev Rheumatol. 2014;10(9):543–51.
Petrillo MG, Ronchetti S, Ricci E, et al. GITR+ regulatory T cells in the treatment of autoimmune diseases. Autoimmun Rev. 2014;14(2):117.
Tao JH, Cheng M, Tang JP, et al. Foxp3, regulatory T cell, and autoimmune diseases. Inflammation. 2016;40(1):1–12.
Bazzazi H, Aghaei M, Memarian A, et al. Th1-Th17 ratio as a new insight in rheumatoid arthritis disease. Iran J Allergy Asthma Immunol. 2018;17(1):68–77.
Yamada H, Nakashima Y, Okazaki K, et al. Preferential accumulation of activated Th1 cells not only in rheumatoid arthritis but also in osteoarthritis joints. J Rheumatol. 2011;38(8):1569–75.
Chemin K, Gerstner C, Malmstrom V. Effector functions of CD4+ T cells at the site of local autoimmune inflammation-lessons from rheumatoid arthritis. Front Immunol. 2019;10:353.
Isoda K, Kamezawa Y, Ayaori M, et al. Osteopontin transgenic mice fed a high-cholesterol diet develop early fatty-streak lesions. Circulation. 2003;107(5):679–81.
Taylor AE, Carey AN, Kudira R, et al. Interleukin 2 promotes hepatic regulatory T cell responses and protects from biliary fibrosis in murine sclerosing cholangitis. Hepatology. 2018;68(5):1905–21.
Acknowledgements
Funding
This work was supported by the Key Research and Development Program of Shanxi Province (No. 201803D31127), funded the study. The journal’s Rapid Service and Open Access Fees was funded by the authors.
Authorship
All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this article, take responsibility for the integrity of the work as a whole, and have given their approval for this version to be published.
Author Contributions
Study design and manuscript writing: JX and JW. Data extraction, quality assessment, analysis and interpretation of data: JH, RJ, XW and HB. All authors were involved in drafting the article or revising it critically for important intellectual content, and all authors approved the final version to be published. Dr. Li had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Disclosures
All authors declare no personal, financial, commercial or academic conflict of interest. Jian-Fang Xie, Jia Wang, Huan-Huan Bai, Jiao-Jiao He, Rui-Huan Jia, Xia Wang, Wen-Qi Zhang, Xiang-Cong Zhao, Xian-Cheng Zhang, Guang-Ying Liu, and Xiao-Feng Li all have nothing to disclose.
Compliance with Ethics Guidelines
This study was approved by the institutional review board of the Second Hospital of Shanxi Medical University (2016 KY-007). The study was performed in accordance with the Helsinki Declaration of 1964 and its later amendments. This was an retrospective study using data which was anonymous, thus patient consent was not required.
Data Availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
Author information
Authors and Affiliations
Corresponding author
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Xie, JF., Wang, J., Bai, HH. et al. A Decreased Absolute Number of Treg Cells in Patients with Active Rheumatoid Arthritis is Associated with Elevated Serum Osteopontin Levels with Disease Progression. Adv Ther 39, 3280–3291 (2022). https://doi.org/10.1007/s12325-022-02171-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12325-022-02171-9