Abstract
Introduction
Newer generation bioresorbable scaffolds (BRSs) with thinner struts and improved deliverability are expected to enhance safety and efficacy profiles. Bioheart (Bio-Heart, Shanghai, China) BRS is constructed from a PLLA (poly-l-lactic acid) backbone coated with a PDLLA (poly d-l-lactic acid) layer eluting sirolimus. We report 2-year serial intracoronary imaging findings.
Methods
In this first-in-human study, 46 patients with single de novo lesions in native coronary vessels (vessel size 3.0–3.75 mm, lesion length ≤ 25 mm) were enrolled at a single institution. Baseline intravascular ultrasound (IVUS) and post-implantation IVUS and optical coherence tomography (OCT) examinations were mandatory. After successful implantations of BRS, the 46 patients were randomized to two different follow-up cohorts in a 2:1 ratio. Thirty patients in cohort 1 had to undergo angiography, IVUS, and OCT follow-ups at 6 and 24 months, respectively. The 16 patients in cohort 2 underwent the same types of imaging follow-ups at 12 and 36 months, respectively. Clinical follow-ups were scheduled uniformly in both cohorts at 1, 6, and 12 months and annually up to 5 years for all patients.
Results
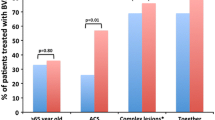
Between August and November 2016, a total of 54 patients were assessed. However, 8 patients could not meet all the inclusion criteria; thus, the remaining 46 patients (age 57.5 ± 8.7 years, 34.8% female, 50.0% with unstable angina, 26.1% diabetics) with 46 target lesions were enrolled in this study. All patients in both cohorts were required to complete clinical follow-up uniformly and regularly. In cohort 1, one patient had definite scaffold thrombosis within 6 months of follow-up; thus, after 6 months, cohort 1 had 96.7% patients . Imaging follow-up was available in 24 patients, and in-scaffold late loss was 0.44 ± 0.47 mm; intracoronary imaging confirmed the late loss was mainly due to to neointimal hyperplasia, but not scaffold recoil.
Conclusions
Serial 2-year clinical and imaging follow-up results confirmed the preliminary safety and efficacy of Bioheart BRS for treatment of simple coronary lesions.





Similar content being viewed by others
References
Pavasini R, Serenelli M, Gallo F, et al. Effectiveness and safety of the ABSORB bioresorbable vascular scaffold for the treatment of coronary artery disease: systematic review and meta-analysis of randomized clinical trials. J Thorac Dis. 2017;9:S887–97.
Ali ZA, Gao R, Kimura T, et al. Three-year outcomes with the absorb bioresorbable scaffold: individual-patient-data meta-analysis from the ABSORB randomized trials. Circulation. 2018;137:464–79.
Serruys PW, Farooq V, Kalesan B, de Vries T, Buszman P, Linke A, Ischinger T, Klauss V, Eberli F, Wijns W, Morice MC, Di Mario C, Corti R, Antoni D, Sohn HY, Eerdmans P, Rademaker- Havinga T, van Es GA, Meier B, Jüni P, Windecker S. Improved safety and reduction in stent thrombosis associated with biodegradable polymer-based biolimus-eluting stents versus durable polymer-based sirolimus-eluting stents in patients with coronary artery disease: final 5-year report of the LEADERS (Limus Eluted From A Durable Versus ERodable Stent Coating) randomized, noninferiority trial. JACC Cardiovasc Interv. 2013;6:777–89.
Zhang Y, Gao R, Xu B, Cummins P, Serruys PW. Bioresorbable scaffolds for coronary artery disease: current status and future prospective. Chin Med J (Engl). 2014;127:1141–8.
Tenekecioglu E, Bourantas C, Abdelghani M, Zeng Y, Silva RC, Tateishi H, Sotomi Y, Onuma Y, Yilmaz M, Serruys PW. From drug eluting stents to bioresorbable scaffolds; to new horizons in PCI. Expert Rev Med Devices. 2016;13:271–86.
Ormiston JA, Serruys PW, Regar E, Dudek D, Thuesen L, Webster MW, Onuma Y, Garcia-Garcia HM, McGreevy R, Veldhof S. A bioabsorbable everolimus-eluting coronary stent system for patients with single de-novo coronary artery lesions (ABSORB): a prospective open-label trial. Lancet. 2008;371:899–907.
Wiebe J, Nef HM, Hamm CW. Current status of bioresorbable scaffolds in the treatment of coronary artery disease. J Am Coll Cardiol. 2014;64:2541–51.
Stone GW, Gao R, Kimura T, Kereiakes DJ, Ellis SG, Onuma Y, Cheong WF, Jones-McMeans J, Su X, Zhang Z, Serruys PW. 1-year outcomes with the Absorb bioresorbable scaffold in patients with coronary artery disease: a patient-level, pooled meta-analysis. Lancet. 2016;387:1277–89.
Verheye S, Ormiston JA, Stewart J, Webster M, Sanidas E, Costa R, Costa JR Jr, Chamie D, Abizaid AS, Pinto I, Morrison L, Toyloy S, Bhat V, Yan J, Abizaid A. A next-generation bioresorbable coronary scaffold system: from bench to first clinical evaluation: 6- and 12-month clinical and multimodality imaging results. JACC Cardiovasc Interv. 2014;7:89–99.
Wu Y, Shen L, Wang Q, et al. Comparison of acute recoil between bioabsorbable poly-l-lactic acid XINSORB scaffold and metallic stent in porcine model. J Biomed Biotechnol. 2012;2012: 413956. https://doi.org/10.1155/2012/413956(Epub2012Oct3).
Gao R, Yang Y, Han Y, Huo Y, Chen J, Yu B, Su X, Li L, Kuo HC, Ying SW, Cheong WF, Zhang Y, Su X, Xu B, Popma JJ, Stone GW, ABSORB China Investigators. Bioresorbable vascular scaffolds versus metallic stents in patients with coronary artery disease: ABSORB China trial. J Am Coll Cardiol. 2015;66:2298–309.
Qiu H, Hu XY, Luo T, Xu B, Xie J, Hu X, Mu CW, Wu C, Tang Y, Ran YM, Xu XL, Chu Y, Gao RL. Short-term safety and effects of a novel fully bioabsorable poly-l-lactic acid sirolimus-eluting stents in porcine coronary arteries. Chin Med J (Engl). 2013;126:1183–5.
Serruys P, De Jaegere P, Bertrand M, Kober G, Marquis JF, Piessens J, Uebis R, Valeix B, Wiegand V. Morphologic change in coronary artery stenosis with the Medtronic Wiktor stent: initial results from the core laboratory for quantitative angiography. Cathet Cardiovasc Diagn. 1991;24:237–45.
Bermejo J, Botas J, Garcia E, Elizaga J, Osende J, Soriano J, Abeytua M, Delcan JL. Mechanisms of residual lumen stenosis after high-pressure stent implantation: a quantitative coronary angiography and intravascular ultrasound study. Circulation. 1998;98:112–8.
Ishibashi Y, Muramatsu T, Nakatani S, Sotomi Y, Suwannasom P, Grundeken MJ, Cho YK, Garcia-Garcia HM, van Boven AJ, Piek JJ, Sabaté M, Helqvist S, Baumbach A, McClean D, de Sousa AM, Wasungu L, Miquel-Hebert K, Dudek D, Chevalier B, Onuma Y, Serruys PW. Incidence and potential mechanism(s) of post-procedural rise of cardiac biomarker in patients with coronary artery narrowing after implantation of an everolimus-eluting bioresorbable vascular scaffold or everolimus- eluting metallic stent. JACC Cardiovasc Interv. 2015;8:1053–63.
Ormiston JA, Serruys PW, Onuma Y, van Geuns RJ, de Bruyne B, Dudek D, Thuesen L, Smits PC, Chevalier B, McClean D, Koolen J, Windecker S, Whitbourn R, Meredith I, Dorange C, Veldhof S, Hebert KM, Rapoza R, Garcia-Garcia HM. First serial assessment at 6 months and 2 years of the second generation of absorb everolimus-eluting bioresorbable vascular scaffold: a multi-imaging modality study. Circ Cardiovasc Interv. 2012;5:620–32.
Taniwaki M, Radu MD, Zaugg S, Amabile N, Garcia- Garcia HM, Yamaji K, Jorgensen E, Kelbaek H, Pilgrim T, Caussin C, Zanchin T, Veugeois A, Abildgaard U, Juni P, Cook S, Koskinas KC, Windecker S, Raber L. Mechanisms of very late drug-eluting stent thrombosis assessed by optical coherence tomography. Circulation. 2016;133:650–60.
Ali ZA, Serruys PW, Kimura T, et al. 2-year outcomes with the Absorb bioresorbable scaffold for treatment of coronary artery disease: a systematic review and meta-analysis of seven randomised trials with an individual patient data sub-study. Lancet. 2017;390:760–72.
Lipinski MJ, Escarcega RO, Baker NC, Benn HA, Gaglia MA Jr, Torguson R, Waksman R. Scaffold thrombosis after percutaneous coronary intervention with ABSORB bioresorbable vascular scaffold: a systematic review and meta-analysis. JACC Cardiovasc Interv. 2016;9:12–24.
Wiebe J, Liebetrau C, Dorr O, Wilkens E, Bauer T, Elsasser A, Achenbach S, Mollmann H, Hamm CW, Nef HM. Impact of the learning curve on procedural results and acute outcome after percutaneous coronary interventions with everolimus-eluting bioresorbable scaffolds in an all-comers population. Cardiovasc Revasc Med. 2015;16:455–60.
Tamburino C, Latib A, van Geuns RJ, Sabate M, Mehilli J, Gori T, Achenbach S, Alvarez MP, Nef H, Lesiak M, Di Mario C, Colombo A, Naber CK, Caramanno G, Capranzano P, Brugaletta S, Geraci S, Araszkiewicz A, Mattesini A, Pyxaras SA, Rzeszutko L, Depukat R, Diletti R, Boone E, Capodanno D, Dudek D. Contemporary practice and technical aspects in coronary intervention with bioresorbable scaffolds: a European perspective. EuroIntervention. 2015;11:45–52.
Acknowledgements
We thank the participants of the study.
Funding
The study was sponsored by Bio-Heart, Shanghai, China, through an institutional research grant. The executive committee, as well as the sponsor, designed the study. The sponsor had no role in data analysis, data interpretation, writing the manuscript, or the decision to submit the manuscript or journal rapid service and Open Access fees for publication.
Authorship
All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this article, take responsibility for the integrity of the work as a whole, and have given their approval for this version to be published.
Author Contributions
Supervision and approval of the clinical trial by Bo Xu and Yongjian Wu.
Data Analysis and interpretation by Changdong Guan, Zhongwei Sun, and Md Misbahul Ferdous.
IVUS data evaluation by Md Misbahul Ferdous, Lijian Gao, Shubi Qiao, and Haibo Liu.
OCT data evaluation by Md Misbahul Ferdous, Fenghuan Hu, Jie Qian, and Hong Qiu.
Angiographic and stenting procedure by Md Misbahul Ferdous, Hongbin Yan, Tong Luo, Weixian Yang, and Yi Mao.
Draft writing by Md Misbahul Ferdous, Jie Zhao, and Lakshme Kottu.
Draft proofreading and supervision of manuscript by Mengyue Yu and Jingang Cui.
Disclosures
MD Misbahul Ferdous, Zhao Jie, Lijian Gao, Shubin Qiao, Haibo Liu, Changdong Guan, Fenghuan Hu, Lakshme Kottu, Jie Qian, Hongbin Yan, Tong Luo, Weixian Yang, Hong Qiu, Yi Mao, Zhongwei Sun, Mengyue Yu, Jingang Cui, Bo Xu, and Yongjian Wu all have nothing to disclose.
Compliance with Ethics Guidelines
This study was approved by the ethics committee at Fuwai Hospital and is in accordance with the principles stated in the Declaration of Helsinki and in line with the International Council on Harmonisation of Technical Requirements for Pharmaceuticals for Human Use guidelines for good clinical practice. An institutional review board at site approved the study. All study participants provided written informed consent before study commencement. The study was registered at ClinicalTrials.gov, identifier NCT02844270.
Data Availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Ferdous, M.M., Jie, Z., Gao, L. et al. A First-in-Human Study of the Bioheart Sirolimus-Eluting Bioresorbable Vascular Scaffold in Patients with Coronary Artery Disease: Two-Year Clinical and Imaging Outcomes. Adv Ther 39, 3749–3765 (2022). https://doi.org/10.1007/s12325-022-02154-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12325-022-02154-w