Abstract
Introduction
This study aims to identify stages of Duchenne muscular dystrophy (DMD) and assess the disease burden by progression stage using real-world administrative claims supplemented by relevant electronic medical record (EMR) data.
Methods
Claims and EMR data from the Decision Resources Group’s Real World Data Repository (2011–2020) were used to identify patients with DMD by diagnosis code and to stratify them into four disease stages by diagnosis and procedure markers reflective of DMD progression. Clinical and medical history data from the Cooperative International Neuromuscular Research Group (CINRG) were used to validate the developed claims-based staging algorithm. The distribution and drivers by disease stage, as well as disease burden, were examined.
Results
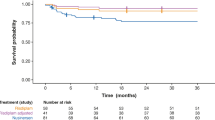
A total of 938 (94%) of patients with DMD identified in claims/EMR data had sufficient information for stage classification. Patients were classified by stage based on patient characteristics and the presence or absence of progression markers such as genetic testing, wheelchair usage, scoliosis treatment, or ventilation assistance. Average ages at stages 1–4 are 7, 13, 18, and 23 years, respectively. Using natural history data, the claims-based staging algorithm was validated with high sensitivity and specificity rates. Both healthcare resource utilization and medical charges increased by stage. For example, the average annualized total charges were $17,688 (stage 1), $36,868 (stage 2), $72,801 (stage 3), and $167,285 (stage 4).
Conclusions
Large-scale claims data supplemented by EMR data can be used to characterize DMD progression and evaluate disease burden which may inform the design of future real-world studies about DMD.
Similar content being viewed by others
References
Duan D, Goemans N, Takeda S, Mercuri E, Aartsma-Rus A. Duchenne muscular dystrophy. Nat Rev Dis Primers. 2021;7(1):13.
Al-Zaidy SA, Lloyd-Puryear M, Kennedy A, Lopez V, Mendell JR. A roadmap to newborn screening for Duchenne muscular dystrophy. Int J Neonatal Screen. 2017;3(2):8.
Connolly AM, Zaidman CM, Golumbek PT, et al. Twice-weekly glucocorticosteroids in infants and young boys with Duchenne muscular dystrophy. Muscle Nerve. 2019;59(6):650–7.
Spehrs-Ciaffi V, Fitting JW, Cotting J, Jeannet PY. Respiratory surveillance of patients with Duchenne and Becker muscular dystrophy. J Pediatr Rehabil Med. 2009;2(2):115–22.
Landfeldt E, Lindgren P, Bell CF, et al. The burden of Duchenne muscular dystrophy: an international, cross-sectional study. Neurology. 2014;83(6):529–36.
Schreiber-Katz O, Klug C, Thiele S, et al. Comparative cost of illness analysis and assessment of health care burden of Duchenne and Becker muscular dystrophies in Germany. Orphanet J Rare Dis. 2014;9:210.
Gloss D, Moxley RT 3rd, Ashwal S, Oskoui M. Practice guideline update summary: corticosteroid treatment of Duchenne muscular dystrophy: report of the guideline development subcommittee of the American Academy of Neurology. Neurology. 2016;86(5):465–72.
PTC Therapeutics. 2014 PTC Therapeutics receives conditional approval in the European Union for Translarna™ for the treatment of nonsense mutation Duchenne muscular dystrophy. http://ir.ptcbio.com/releasedetail.cfm?releaseid=863914. Accessed 6 Mar 2022.
US Food and Drug Administration. FDA grants accelerated approval to first targeted treatment for rare Duchenne muscular dystrophy mutation. https://www.fda.gov/news-events/press-announcements/fda-grants-accelerated-approval-first-targeted-treatment-rare-duchenne-muscular-dystrophy-mutation. Accessed 6 Mar 2022.
Sheehan DW, Birnkrant DJ, Benditt JO, et al. Respiratory management of the patient with Duchenne muscular dystrophy. Pediatrics. 2018;142(Suppl 2):S62–71.
Case LE, Apkon SD, Eagle M, et al. Rehabilitation management of the patient with Duchenne muscular dystrophy. Pediatrics. 2018;142(Suppl 2):S17–33.
Koeks Z, Bladen CL, Salgado D, et al. Clinical outcomes in Duchenne muscular dystrophy: a study of 5345 patients from the TREAT-NMD DMD global database. J Neuromuscul Dis. 2017;4(4):293–306.
Fujino H, Iwata Y, Saito T, Matsumura T, Fujimura H, Imura O. The experiences of patients with Duchenne muscular dystrophy in facing and learning about their clinical conditions. Int J Qual Stud Health Well-being. 2016;11:32045.
Muntoni F, Domingos J, Manzur AY, et al. Categorising trajectories and individual item changes of the North Star ambulatory assessment in patients with Duchenne muscular dystrophy. PLoS One. 2019;14(9):e0221097.
Goemans N, Wong B, Van den Hauwe M, et al. Prognostic factors for changes in the timed 4-stair climb in patients with Duchenne muscular dystrophy, and implications for measuring drug efficacy: a multi-institutional collaboration. PLoS One. 2020;15(6):e0232870.
Goemans N, Signorovitch J, Sajeev G, et al. Suitability of external controls for drug evaluation in Duchenne muscular dystrophy. Neurology. 2020;95(10):e1381–91.
Marden JR, Freimark J, Yao Z, Signorovitch J, Tian C, Wong BL. Real-world outcomes of long-term prednisone and deflazacort use in patients with Duchenne muscular dystrophy: experience at a single, large care center. J Comp Eff Res. 2020;9(3):177–89.
Bonifati MD, Ruzza G, Bonometto P, et al. A multicenter, double-blind, randomized trial of deflazacort versus prednisone in Duchenne muscular dystrophy. Muscle Nerve. 2000;23(9):1344–7.
Griggs RC, Miller JP, Greenberg CR, et al. Efficacy and safety of deflazacort vs prednisone and placebo for Duchenne muscular dystrophy. Neurology. 2016;87(20):2123–31.
Bello L, Gordish-Dressman H, Morgenroth LP, et al. Prednisone/prednisolone and deflazacort regimens in the CINRG Duchenne natural history study. Neurology. 2015;85(12):1048–55.
McDonald CM, Henricson EK, Abresch RT, et al. Long-term effects of glucocorticoids on function, quality of life, and survival in patients with Duchenne muscular dystrophy: a prospective cohort study. Lancet. 2018;391(10119):451–61.
McDonald CM, Henricson EK, Abresch RT, et al. The Cooperative International Neuromuscular Research Group Duchenne natural history study–a longitudinal investigation in the era of glucocorticoid therapy: design of protocol and the methods used. Muscle Nerve. 2013;48(1):32–54.
Centers for Medicare & Medicaid Services. 2021 ICD-10-CM. https://www.cms.gov/medicare/icd-10/2021-icd-10-cm. Accessed 6 Mar 2022.
Landfeldt E, Alfredsson L, Straub V, Lochmuller H, Bushby K, Lindgren P. Economic evaluation in Duchenne muscular dystrophy: model frameworks for cost-effectiveness analysis. Pharmacoeconomics. 2017;35(2):249–58.
Liao KP, Cai T, Gainer V, et al. Electronic medical records for discovery research in rheumatoid arthritis. Arthritis Care Res (Hoboken). 2010;62(8):1120–7.
Mirelman A, Ben Or Frank M, Melamed M, et al. Detecting sensitive mobility features for Parkinson's disease stages via machine learning. Mov Disord. 2021;36(9):2144–55.
Benchimol EI, Guttmann A, Mack DR, et al. Validation of international algorithms to identify adults with inflammatory bowel disease in health administrative data from Ontario. Canada J Clin Epidemiol. 2014;67(8):887–96.
Hebert PL, Geiss LS, Tierney EF, Engelgau MM, Yawn BP, McBean AM. Identifying persons with diabetes using Medicare claims data. Am J Med Qual. 1999;14(6):270–7.
Teoh LJ, Geelhoed EA, Bayley K, Leonard H, Laing NG. Health care utilization and costs for children and adults with Duchenne muscular dystrophy. Muscle Nerve. 2016;53(6):877–84.
Thayer S, Bell C, McDonald CM. The direct cost of managing a rare disease: assessing medical and pharmacy costs associated with Duchenne muscular dystrophy in the United States. J Manag Care Spec Pharm. 2017;23(6):633–41.
Stott-Miller M, Vlahiotis A, Palmer L. Treatment patterns, resource utilization and costs in muscular dystrophy patients: analysis using administrative claims data (Abstract). Value Health. 2015;18(7):PA764-A5.
Ryder S, Leadley RM, Armstrong N, et al. The burden, epidemiology, costs and treatment for Duchenne muscular dystrophy: an evidence review. Orphanet J Rare Dis. 2017;12(1):79.
Mayer O, Karafilidis J, Higgins K, Griffin B. Descriptive characteristics of males with Duchenne muscular dystrophy using insurance claims data. Neuromuscul Disord. 2018;28(S2):S39.
Acknowledgements
We acknowledge the help from Kai (Kristy) Sheng on data analysis for the study and Gloria DeWalt on drafting of the manuscript. Both Kristy and Gloria are employees of Analysis Group and their assistance is covered by the funding from Sarepta Therapeutics, Inc. This study also acknowledges the efforts and contributions from CINRG investigators.
Funding
This study and the Rapid Service Fee for the journal were funded by Sarepta Therapeutics, Inc.
Authorship
All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this article, take responsibility for the integrity of the work as a whole, and have given their approval for this version to be published. All authors (Joel Iff, Yi Zhong, Deepshekhar Gupta, Xander Paul, Edward Tuttle, Erik Henricson, Rachel Schrader and CINRG DNHS Investigators) have agreed the contents of the submission.
Author Contributions
Joel Iff, Yi Zhong, Deepshekhar Gupta, Xander Paul, Edward Tuttle, Erik Henricson, and Rachel Schrader participated in the study design, interpretation of results, and review of this manuscript. Yi Zhong, Xander Paul, Deepshekhar Gupta, Edward Tuttle and CINRG DNHS Investigators coordinated the data collection. Yi Zhong, Xander Paul, Deepshekhar Gupta and Edward Tuttle conducted the data analysis.
List of Investigators
Cooperative International Neuromuscular Research Group (CINRG) Investigators: V. Vishwanathan (Sundaram Medical Foundation and Apollo Children’s Hospital, Chennai, India); S. Chidambaranathan (Sundaram Medical Foundation and Apollo Children’s Hospital, Chennai, India); W. Douglas Biggar (Holland Bloorview Kids Rehab Hospital, Toronto, ON, Canada); Laura C. McAdam (Holland Bloorview Kids Rehab Hospital, Toronto, ON, Canada); Jean K. Mah (Alberta Children’s Hospital, Calgary, AB, Canada); Mar Tulinius (Queen Silvia Children’s Hospital, Göteborg, Sweden); Avital Cnaan (Children’s National Medical Center, Washington DC, USA); Lauren P. Morgenroth (Children’s National Medical Center, Washington DC, USA); Robert Leshner (Children’s National Medical Center, Washington DC, USA); Carolina TesiRocha (Children’s National Medical Center, Washington DC, USA); Mathula Thangarajh (Children’s National Medical Center, Washington DC, USA); Tina Duong (Children’s National Medical Center, Washington DC, USA); Andrew Kornberg (Royal Children’s Hospital, Melbourne, Australia); Monique Ryan (Royal Children’s Hospital, Melbourne, Australia);Yoram Nevo (Hadassah Hebrew University Hospital, Jerusalem, Israel); Alberto Dubrovsky (Instituto de Neurosciencias Fundacion Favaloro, Buenos Aires, Argentina); Paula R. Clemens (University of Pittsburgh and Department of Veterans Affairs, Pittsburgh, PA, USA); Hoda Abdel-Hamid (University of Pittsburgh and Department of Veterans Affairs, Pittsburgh, PA, USA); Anne M. Connolly (Washington University in St Louis, St Louis, MO, USA); Alan Pestronk (Washington University in St Louis, St Louis, MO, USA); Jean Teasley (Children’s Hospital of Virginia, Richmond, VA, USA); Tulio E. Bertorini (University of Tennessee, Memphis, TN, USA); Richard Webster (Children’s Hospital at Westmead, Sydney, Australia); Hanna Kolski (University of Alberta, Edmonton, AB, Canada); Nancy Kuntz (Mayo Clinic, Rochester, MN, USA); Sherilyn Driscoll (Mayo Clinic, Rochester, MN, USA); John B. Bodensteiner (Mayo Clinic, Rochester, MN, USA); Jose Carlo (University of Puerto Rico, San Juan, Puerto, Rico); Ksenija Gorni (University of Pavia and Niguarda Ca’ Granda Hospital, Milan, Italy); Timothy Lotze (Texas Children’s Hospital, Houston, TX, USA); John W. Day (University of Minnesota, Minneapolis, MN, USA); Peter Karachunski (University of Minnesota, Minneapolis, MN, USA); Erik K. Henricson (University of California, Davis, CA, USA); Richard T. Abresch (University of California, Davis, CA, USA); Nanette C. Joyce (University of California, Davis, CA, USA); Craig M. McDonald (University of California, Davis, CA, USA). Duchenne Natural History Study was funded through grants from the US Department of Education/National Institute on Disability and Rehabilitation Research (H133B031118 and H133B090001), US Department of Defense (W81XWH-09-1-0592), the National Institutes of Health (UL1RR Q12 031,988, U54HD053177, UL1RR024992, U54RR026139, G12RR003051, 1R01AR061875, and RO1AR062380), and Parent Project Muscular Dystrophy. The authors thank the participating patients and their families for taking part in this research and gratefully acknowledge the participation of all investigators, clinical coordinators, clinical evaluator trainers, clinical evaluators, coordinators, and supporting staff for their contributions to the study.
Prior Presentation
This study was previously presented at the 25th International Annual Congress of the World Muscle Society, September 30–October 4, 2020 (virtual).
Disclosures
Yi Zhong, Xander Paul, Deepshekhar Gupta, and Edward Tuttle are employees of Analysis Group, Inc. and received funding from Sarepta Therapeutics Inc. for conducting the analysis and writing the manuscript. Xander Paul has started to work at Intensity, LLC during the completion of the manuscript. Joel Iff is an employee of Sarepta Therapeutics, Inc. and may own stock/stock options in the company. Rachel Schrader is an employee of Parent Project Muscular Dystrophy (PPMD). PPMD received consulting fee from Sarepta Therapeutics, Inc for this study. Erik Henricson reports consulting fees from Sarepta Therapeutics, Inc.
Compliance with Ethics Guidelines
For CINRG data (NCT00468832, https://cinrgresearch.org/), the institutional or ethics review boards at each participating institution approved the study protocol and the consent/assent documents. Informed consent/assent was obtained from each participant or caregiver as appropriate prior to conducting the study procedures. DRG data do not require institutional review board review as it only contains de-identified data. The authors have obtained permission to access and use the data from the owners of the data.
Data Availability
The data sets generated during and/or analyzed during the current study are not publicly available due to data usage agreement between DRG, CINRG, and Sarepta.
Author information
Authors and Affiliations
Consortia
Corresponding author
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Iff, J., Zhong, Y., Gupta, D. et al. Disease Progression Stages and Burden in Patients with Duchenne Muscular Dystrophy Using Administrative Claims Supplemented by Electronic Medical Records. Adv Ther 39, 2906–2919 (2022). https://doi.org/10.1007/s12325-022-02117-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12325-022-02117-1