Abstract
Introduction
Number needed to treat (NNT) estimates are a practical metric to help identify the most effective therapies. Our objective is to compare 11 biologic drugs for moderate-to-severe psoriasis in terms of NNT.
Methods
The NNT data were obtained from a Bayesian network meta-analysis of 42 double-blind, randomized, phase 3 clinical trials for 11 biologics (adalimumab, brodalumab, certolizumab pegol, etanercept, guselkumab, infliximab, ixekizumab, risankizumab, secukinumab, tildrakizumab, and ustekinumab). We determined NNT to achieve Psoriasis Area and Severity Index (PASI) 75/90/100 responses at weeks 4, 8, 12, 16, and 48/52 and Dermatology Life Quality Index (DLQI) response 0, 1 at week 12.
Results
Highest efficacy (lowest NNT) was with brodalumab and ixekizumab for PASI 90 at weeks 4, 8, and 12; ixekizumab for PASI 90/100 at week 16; and brodalumab for PASI 100 at week 12. After 48/52 weeks, risankizumab had the highest efficacy for PASI 90/100 overlapping with guselkumab, brodalumab, and ixekizumab for PASI 90 and with brodalumab and ixekizumab for PASI 100. Ixekizumab had the highest efficacy for DLQI (0,1) at week 12.
Conclusions
Brodalumab and ixekizumab had the lowest NNTs for achieving PASI responses at early time points and were not significantly different than risankizumab and guselkumab after 48/52 weeks.
Similar content being viewed by others
Why carry out this study? |
Number needed to treat (NNT) estimations provide a simple and practical metric for payors and providers to compare available biologic treatments for moderate-to-severe psoriasis |
A network meta-analysis of 42 clinical trials for 11 biologic drugs was used to calculate NNT |
A lower NNT indicates that a treatment is more effective than the comparator |
What was learned from the study? |
Brodalumab and ixekizumab had the highest efficacy (lowest NNT) for achieving PASI 75/90/100 responses at early time points (week 4 to week 16). After 48/52 weeks, risankizumab, guselkumab, brodalumab, and ixekizumab had the lowest NNT and were not significantly different. Ixekizumab had the highest efficacy in quality-of-life improvement (achievement of Dermatology Life Quality Index response 0,1 at week 12) |
Digital Features
This article is published with digital features, including animated graphs, to facilitate understanding of the article. To view digital features for this article go to https://doi.org/10.6084/m9.figshare.19323539.
Introduction
Psoriasis is a chronic disease characterized by painful, itchy skin lesions, which can greatly impact a patient’s quality of life [1,2,3]. Currently, 11 biologics have been approved by the United States Food and Drug Administration (FDA) for the treatment of moderate-to-severe psoriasis, which are anti-interleukin (IL)-17 agents (brodalumab, ixekizumab, and secukinumab), an anti-IL-12/-23 agent (ustekinumab), anti-IL-23 agents (guselkumab, risankizumab, and tildrakizumab), and anti-tumor necrosis factor (TNF) agents (adalimumab, certolizumab pegol, etanercept, and infliximab). Health care providers and patients benefit from an assessment of comparative efficacy to help identify the most effective and appropriate therapy. Network meta-analyses (NMA) are indirect comparisons of individual treatments versus placebo, and while NMAs have been conducted in the short- and long-term [4,5,6,7,8], few comprehensive long-term NMAs have focused on the number needed to treat (NNT) [9,10,11]. Number needed to treat is the average number of patients required to be treated with a given treatment to achieve an outcome in question. Number needed to treat estimates provide a simple and practical metric for payors and providers to compare available treatments, as a lower NNT indicates the treatment is more effective than the comparator. Comparative NNTs provide more comprehensive evidence for dermatologists in their treatment decisions.
The objective of this study is to provide a comprehensive long-term NMA focused on NNT to compare efficacy among 11 FDA-approved biologics for the treatment of moderate-to-severe psoriasis. Bayesian NMA (BNMA) was used to calculate NNT for achieving skin improvement (measured as Psoriasis Area and Severity Index [PASI] 75, PASI 90, and PASI 100 responses at weeks 4, 8, 12, 16, and 48/52) and quality-of-life improvement (measured as achievement of Dermatology Life Quality Index [DLQI] response of 0, 1 at week 12). This study uniquely includes rapid PASI response rates at weeks 4 and 8 and quality of life in terms of NNT.
Methods
Efficacy data for this NMA analysis were collected from 42 phase 3 double-blind randomized clinical trials for 11 approved biologic treatments for moderate-to-severe psoriasis: anti-IL-17 agents brodalumab, ixekizumab, and secukinumab; anti-IL-12/-23 agent ustekinumab; anti-IL-23 agents guselkumab, risankizumab, and tildrakizumab; and anti-TNF agents adalimumab, certolizumab pegol, etanercept, and infliximab [12,13,14,15,16,17,18,19,20,21,22,23,24,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46]. These studies included patients who were ≥ 18 years of age with moderate-to-severe psoriasis, and inclusion and exclusion criteria for the studies have been previously reported. Studies used in each analysis of the NMA are in Supplementary Material Table S1.
This article is based on previously conducted studies and does not contain any studies with human participants or animals performed by any of the authors.
Outcome measures extracted for short- and long-term skin improvement were achievement of PASI 75, PASI 90, and PASI 100 at weeks 4, 8, 12, 16, and 48/52 in terms of the NNT. The outcome measure for quality-of-life improvement was achievement of DLQI (0,1) at week 12 in terms of NNT, defined as no impact or minimal impact on quality of life. Dermatology Life Quality Index (0,1) data were not available after week 12 from enough randomized controlled trials (RCTs) for NMA inclusion; thus, additional time points were not evaluated.
Systematic Literature Review
Efficacy data on outcome measures were obtained from a systematic literature review previously reported by Warren et al. [4], which is updated in this report. Briefly, a search was performed using the OvidSP platform for literature published between January 1, 1990, and August 21, 2020. The search parameters were designed to identify publications that reported data from phase 3 RCTs of biologics approved for the treatment of moderate-to-severe psoriasis. Studies included in the NMA are listed above and in Supplementary Material Table S1. The Cochrane Handbook for Systematic Reviews of Interventions guidance was followed [47].
In this update of the previous report [4], long-term analyses were included to week 48/52. All doses were included in the analyses (shown in the evidence network for NMA in Fig. 1). Our data present only FDA-approved label doses, as was done in the previous report [4]. Treatments/studies were excluded from any specific analysis where data were not available. For long-term efficacy analysis (week 48/52), studies with re-randomization at the end of the induction period (week 12/16) were excluded. The ustekinumab arm in AMAGINE-2,3 was excluded for week 48/52 analysis; data were not available for patients who were taking ustekinumab and did not receive a brodalumab rescue dose at week 16.
Evidence network for NMA of PASI 90 at week 12. A total of 35 RCTs and 20 treatments are included. Lines represent direct comparisons using RCTs (n). Numbers and thicknesses of lines represent the number of RCTs included in each comparison (N). a200 mg at Weeks 0 and 4, then every 12 weeks; b100 mg at Weeks 0 and 4, then every 12 weeks; c100 mg at Weeks 0 and 4, then every 8 weeks; d80 mg at Week 0, 40 mg at week 1, then 40 mg Q2W. ADA adalimumab (anti-TNF agent), BIW twice weekly, BRO brodalumab (anti-IL-17 agent), CZP certolizumab pegol (anti-TNF agent), ETN etanercept (anti-TNF agent), GUS guselkumab (anti-IL-23 agent), IFX infliximab (anti-TNF agent), IXE ixekizumab (anti-IL-17 agent), NMA network meta-analysis, PASI Psoriasis Area and Severity Index, Q2W every 2 weeks, Q4W every 4 weeks, Q8W every 8 weeks, Q12W every 12 weeks, RCT randomized controlled trial, RIS risankizumab (anti-IL-23 agent), SEC secukinumab (anti-IL-17 agent), TIL tildrakizumab (anti-IL-23 agent), UST ustekinumab (anti-IL-12/-23 agent)
Statistical Analyses
Bayesian NMAs were performed on PASI 75, PASI 90, and PASI 100 response rates at different time points, separately, using normal approximation. A mixed effect model was assumed with fixed treatment effect and random baseline effect to allow various placebo response rates across different studies. Non-informative priors were used for the model parameters. The BNMA describes the joint probability distribution of these response variables, and NNTs were calculated using the response difference of each respective treatment versus placebo. Posterior distributions of the treatment effect were presented by posterior samples and summarized by posterior means and 95% credible intervals. Bayesian NMAs were conducted through an internal tool based on JAGS and R with three chains; a thinning factor of 50; burn-in iterations of 300,000; and 1,000,000 total samples. Posterior samples of NNTs to achieve PASI 75, 90, and 100 responses were obtained as reciprocals of the posterior samples of the corresponding treatment effects. Posterior means and 95% credible intervals were also summarized for NNTs.
Results
The evidence network for the NMA of PASI 90 response at week 12 is presented in Fig. 1. This is representative of evidence network findings for NMA for PASI response at other time points and DLQI (0,1) at week 12. All studies included in each analysis of the NMA are in the Supplementary Material (Table S1).
Short-term Efficacy and Quality of Life in Terms of NNT
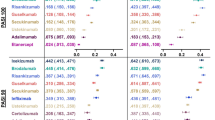
Brodalumab and ixekizumab had the highest efficacy (lowest NNT) to achieve PASI 90 and PASI 100 at weeks 4 and 8 (Table 1), the earliest time points examined in this analysis. At week 12, the lowest NNT to achieve PASI 90 was with ixekizumab (1.44, 95% credible interval [Crl] 1.39, 1.49) with density distribution overlapping with brodalumab (1.49, 95% Crl 1.43, 1.55), and the lowest NNT to achieve PASI 100 was with brodalumab (2.50, 95% Crl 2.35, 2.67) with density distribution overlapping with ixekizumab (2.61, 95% Crl 2.45, 2.79) (Fig. 2 and Table 1). At week 16, the lowest NNT to achieve PASI 90 was with ixekizumab (1.38, 95% Crl 1.30, 1.47) with density distribution overlapping with risankizumab (1.40, 95% Crl 1.34, 1.46), and the lowest NNT to achieve PASI 100 was with ixekizumab (2.25, 95% Crl 2.04, 2.50) with density distribution overlapping with brodalumab (2.29, 95% Crl 2.04, 2.60) (Fig. 2; Table 1). Estimated mean treatment effects (relative to placebo) on PASI 90 and PASI 100 response rates are presented at weeks 12 and 16 in Fig. 3. Number needed to treat to achieve PASI 75 and estimated mean treatment effects (relative to placebo) on PASI 75 response rates are presented at weeks 4, 8, 12, and 16 in the Supplementary Material (Figure S1, Figure S2, and Table S2).
NNT to achieve PASI 90 and PASI 100 responses at weeks 12, 16, and 48/52. Data are mean relative to placebo. Missing data are marked NA. Studies included in the analysis are listed in the Supplementary Material. ADA adalimumab, BRO brodalumab, Crl credible interval, CZP certolizumab pegol, ETN etanercept, GUS guselkumab, IFX infliximab, IXE ixekizumab, NA not applicable, NNT number needed to treat, PASI Psoriasis Area and Severity Index, RIS risankizumab, SEC secukinumab, TIL tildrakizumab, UST ustekinumab
Treatment effects on PASI 90 and 100 response rates at week 12, week 16, and week 48/52. Data are mean relative to placebo. Missing data are marked NA. Studies included in the analysis are listed in the Supplementary Material. ADA adalimumab, BRO brodalumab, CZP certolizumab pegol, ETN etanercept, GUS guselkumab, IFX infliximab, IXE ixekizumab, NA not applicable, PASI Psoriasis Area and Severity Index, RIS risankizumab, SEC secukinumab, TIL tildrakizumab, UST ustekinumab
At week 12, the lowest NNT to achieve DLQI (0,1) was with ixekizumab (1.75, Crl 1.63, 1.87) followed by and overlapping in distribution with brodalumab (1.83, Crl 1.74, 1.93) and secukinumab (1.88, Crl 1.76, 2.01) (Supplementary Material Table S3).
Long-term Efficacy in Terms of NNT
After 48/52 weeks, the lowest NNT to achieve PASI 90 was with risankizumab (1.27, 95% Crl 1.21, 1.34) with density distribution overlapping with guselkumab (1.32, 95% Crl 1.26, 1.39), brodalumab (1.40, 95% Crl 1.31, 1.50), and ixekizumab (1.42, 95% Crl 1.33, 1.52) (Figs. 2, 4, and Table 1). The lowest NNT to achieve PASI 100 was with risankizumab (1.77, 95% Crl 1.64, 1.90) with density distribution overlapping with brodalumab (1.85, 95% Crl 1.68, 2.05) and ixekizumab (1.90, 95% Crl 1.74, 2.07) (Fig. 2; Table 1). Estimated mean treatment effects (relative to placebo) on PASI 90 and PASI 100 response rates are presented at week 48/52 in Fig. 3. Number needed to treat to achieve PASI 75 and estimated mean treatment effects (relative to placebo) on PASI 75 response rates are presented at week 48/52 (in the the Supplementary Material: Figure S1, Figure S2, and Table S2).
a Relative response of biologic treatments of moderate-to-severe plaque psoriasis based on BNMA for PASI 90 at week 48/52. b NNT to achieve PASI 90 at week 48/52. PASI 100 data are in panels (c) and (d). Missing data are marked NA. Studies included in the analysis from the studies are listed in the Supplementary Material. ADA adalimumab, BNMA Bayesian network meta-analysis, BRO brodalumab, Crl credible interval, CZP certolizumab pegol, ETN etanercept, GUS guselkumab, IFX infliximab, IXE ixekizumab, NA not applicable, NNT number needed to treat, PASI Psoriasis Area and Severity Index, RIS risankizumab, SEC secukinumab, TIL tildrakizumab, UST ustekinumab
Network meta-analysis is comprehensive in nature and examines multiple time points over induction periods plus longer term follow-up to 1 year (48/52 weeks). Animations are provided to help the reader more fully understand these complex data. Animated videos are in the online/HTML version of the manuscript or follow the digital features link under the abstract (Video 1, relative response [proportion] based on NMA for PASI 90; Video 2, NNT to achieve PASI 90; Video 3, relative response [proportion] based on NMA for PASI 100; Video 4, NNT to achieve PASI 100).
Discussion
Out of all treatments examined and consistent with previous analysis [9, 10, 48, 49], ixekizumab and brodalumab had the lowest NNTs for achievement of completely clear or nearly completely clear skin (PASI 100 and 90 responses) at early time points (as early as weeks 4 and 8) and at weeks 12 and 16 indicating rapid clearance of plaque psoriasis versus other biologic treatments for patients with moderate-to-severe psoriasis. Consistent with previous NMAs [4], there were significant differences in the performance of members within the same class. For example, brodalumab and ixekizumab had consistently lower NNTs (higher efficacy) versus secukinumab with the exception of achievement of DLQI (0,1) at week 12 where distributions overlapped. After 48/52 weeks, ixekizumab and brodalumab were comparable to anti-IL-23 agents with density distributions overlapping with risankizumab (the treatment with the lowest NNT) for PASI 90 and PASI 100 and guselkumab for PASI 90. This was consistent with a head-to-head study of ixekizumab and guselkumab that showed similar levels of complete skin clearance (PASI 100) at week 24 [50]. Anti-TNF agents had the highest NNTs (lowest efficacy) across all analyses.
Our report aligns with previous NMA findings focused on NNT [6, 9,10,11] but uniquely includes earlier time points (weeks 4 and 8) and quality of life (DLQI 0,1 at week 12). Compared to recent NMA with NNT estimates [9], the BNMA statistical methodology used here required fewer assumptions than other analytical methodologies, such as ordinal BNMA. We included only phase 3 RCTs, which allowed for more homogeneous comparisons between treatments and have distinct time points at weeks 4, 8, 12, and 16 (which is unique from other publications that employed a range of time points).
Several limitations, common to all meta-analyses, should be considered. An overview of NMA is available in the following online supplement [4]. As previously described, the strength of NMA is that it is a statistical methodology that allows for both direct and indirect evidence for comparison of many treatments at once, but it relies on published studies with differing specific imputation methods, study designs, and patient characteristics. The number of clinical trials is also different for each biologic drug, which might affect the results of the study. Treatment expectations and treatment goals have also evolved over time since the first psoriasis treatments were approved, and this contributes additional heterogeneity when including present day RCTs and older clinical trials within the NMA.
Patients prefer complete clearance of psoriasis, and PASI 100 is now an attainable treatment goal [51]. In this NMA focused on NNT, brodalumab and ixekizumab had the highest efficacy (lowest NNTs) in complete clearance of psoriasis as early as weeks 4 and 8 of treatment and at weeks 12 and 16. After 1 year, there was overlap in distribution in NNT to achieve PASI 100 between risankizumab (with the lowest NNT), broadlumab, and ixekizumab indicating that these biologics were not significantly different after 1 year (weeks 48/52).
References
Mattei PL, Corey KC, Kimball AB. Psoriasis Area Severity Index (PASI) and the Dermatology Life Quality Index (DLQI): the correlation between disease severity and psychological burden in patients treated with biological therapies. J Eur Acad Dermatol Venereol. 2014;28(3):333–7.
Warren RB, Kleyn CE, Gulliver WP. Cumulative life course impairment in psoriasis: patient perception of disease-related impairment throughout the life course. Br J Dermatol. 2011;164(Suppl 1):1–14.
Lebwohl MG, Bachelez H, Barker J, et al. Patient perspectives in the management of psoriasis: results from the population-based Multinational Assessment of Psoriasis and Psoriatic Arthritis Survey. J Am Acad Dermatol. 2014;70(5):871–81.e1–30.
Warren RB, See K, Burge R, et al. Rapid response of biologic treatments of moderate-to-severe plaque psoriasis: a comprehensive investigation using bayesian and frequentist network meta-analyses. Dermatol Ther (Heidelb). 2020;10(1):73–86.
Bai F, Li GG, Liu Q, et al. J Immunol Res. 2019. https://doi.org/10.1155/2019/2546161.
Sawyer LM, Malottki K, Sabry-Grant C, et al. Assessing the relative efficacy of interleukin-17 and interleukin-23 targeted treatments for moderate-to-severe plaque psoriasis: A systematic review and network meta-analysis of PASI response. PLoS One. 2019;14(8): e0220868.
Warren RB, Gooderham M, Burge R, et al. Comparison of cumulative clinical benefits of biologics for the treatment of psoriasis over 16 weeks: results from a network meta-analysis. J Am Acad Dermatol. 2020;82(5):1138–49.
Sawyer LM, Cornic L, Levin L, et al. Long-term efficacy of novel therapies in moderate-to-severe plaque psoriasis: a systematic review and network meta-analysis of PASI response. J Eur Acad Dermatol Venereol. 2019;33(2):355–66.
Armstrong AW, Soliman AM, Betts KA, et al. Comparative efficacy and relative ranking of biologics and oral therapies for moderate-to-severe plaque psoriasis: a network meta-analysis. Dermatol Ther (Heidelb). 2021;11(3):885–905.
Armstrong AW, Puig L, Joshi A, et al. Comparison of biologics and oral treatments for plaque psoriasis: a meta-analysis. JAMA Dermatol. 2020;156(3):258–69.
Yasmeen N, Sawyer LM, Malottki K, et al. Targeted therapies for patients with moderate-to-severe psoriasis: a systematic review and network meta-analysis of PASI response at 1 year. J Dermatolog Treat. 2020. https://doi.org/10.1080/09546634.2020.1743811.
Papp KA, Reich K, Paul C, et al. A prospective phase III, randomized, double-blind, placebo-controlled study of brodalumab in patients with moderate-to-severe plaque psoriasis. Br J Dermatol. 2016;175(2):273–86.
Lebwohl M, Strober B, Menter A, et al. Phase 3 studies comparing brodalumab with ustekinumab in psoriasis. N Engl J Med. 2015;373(14):1318–28.
Griffiths CE, Reich K, Lebwohl M, et al. Comparison of ixekizumab with etanercept or placebo in moderate-to-severe psoriasis (UNCOVER-2 and UNCOVER-3): results from two phase 3 randomised trials. Lancet. 2015;386(9993):541–51.
Gordon KB, Blauvelt A, Papp KA, et al. Phase 3 trials of ixekizumab in moderate-to-severe plaque psoriasis. N Engl J Med. 2016;375(4):345–56.
Langley RG, Papp K, Gooderham M, et al. Efficacy and safety of continuous every-2-week dosing of ixekizumab over 52 weeks in patients with moderate-to-severe plaque psoriasis in a randomized phase III trial (IXORA-P). Br J Dermatol. 2018;178(6):1315–23.
Reich K, Pinter A, Lacour JP, et al. Comparison of ixekizumab with ustekinumab in moderate-to-severe psoriasis: 24-week results from IXORA-S, a phase III study. Br J Dermatol. 2017;177(4):1014–23.
Paul C, Griffiths CEM, van de Kerkhof PCM, et al. Ixekizumab provides superior efficacy compared with ustekinumab over 52 weeks of treatment: results from IXORA-S, a phase 3 study. J Am Acad Dermatol. 2019;80(1):70-79.e3.
Langley RG, Elewski BE, Lebwohl M, et al. Secukinumab in plaque psoriasis–results of two phase 3 trials. N Engl J Med. 2014;371(4):326–38.
Blauvelt A, Prinz JC, Gottlieb AB, et al. Secukinumab administration by pre-filled syringe: efficacy, safety and usability results from a randomized controlled trial in psoriasis (FEATURE). Br J Dermatol. 2015;172(2):484–93.
Paul C, Lacour JP, Tedremets L, et al. Efficacy, safety and usability of secukinumab administration by autoinjector/pen in psoriasis: a randomized, controlled trial (JUNCTURE). J Eur Acad Dermatol Venereol. 2015;29(6):1082–90.
Thaci D, Blauvelt A, Reich K, et al. Secukinumab is superior to ustekinumab in clearing skin of subjects with moderate to severe plaque psoriasis: CLEAR, a randomized controlled trial. J Am Acad Dermatol. 2015;73(3):400–9.
Bagel J, Nia J, Hashim PW, et al. Secukinumab is superior to ustekinumab in clearing skin in patients with moderate to severe plaque psoriasis (16-week CLARITY results). Dermatol Ther (Heidelb). 2018;8(4):571–9.
Reich K, Armstrong AW, Langley RG, et al. Guselkumab versus secukinumab for the treatment of moderate-to-severe psoriasis (ECLIPSE): results from a phase 3, randomised controlled trial. Lancet. 2019;394(10201):831–9.
Mrowietz U, Leonardi CL, Girolomoni G, et al. Secukinumab retreatment-as-needed versus fixed-interval maintenance regimen for moderate to severe plaque psoriasis: A randomized, double-blind, noninferiority trial (SCULPTURE). J Am Acad Dermatol. 2015;73(1):27-36.e1.
Griffiths CE, Strober BE, van de Kerkhof P, et al. Comparison of ustekinumab and etanercept for moderate-to-severe psoriasis. N Engl J Med. 2010;362(2):118–28.
Leonardi CL, Kimball AB, Papp KA, et al. Efficacy and safety of ustekinumab, a human interleukin-12/23 monoclonal antibody, in patients with psoriasis: 76-week results from a randomised, double-blind, placebo-controlled trial (PHOENIX 1). Lancet. 2008;371(9625):1665–74.
Papp KA, Langley RG, Lebwohl M, et al. Efficacy and safety of ustekinumab, a human interleukin-12/23 monoclonal antibody, in patients with psoriasis: 52-week results from a randomised, double-blind, placebo-controlled trial (PHOENIX 2). Lancet. 2008;371(1675):1675–84.
Tsai TF, Ho JC, Song M, et al. Efficacy and safety of ustekinumab for the treatment of moderate-to-severe psoriasis: a phase III, randomized, placebo-controlled trial in Taiwanese and Korean patients (PEARL). J Dermatol Sci. 2011;63(3):154–63.
CR015166 curated database, ustekinumab. Clinicaltrials.gov identification number NCT00723528. Data on file, Eli Lilly and Company.
Gordon KB, Strober B, Lebwohl M, et al. Efficacy and safety of risankizumab in moderate-to-severe plaque psoriasis (UltIMMa-1 and UltIMMa-2): results from two double-blind, randomised, placebo-controlled and ustekinumab-controlled phase 3 trials. Lancet. 2018;392(10148):650–61.
Blauvelt A, Papp KA, Griffiths CE, et al. Efficacy and safety of guselkumab, an anti-interleukin-23 monoclonal antibody, compared with adalimumab for the continuous treatment of patients with moderate to severe psoriasis: results from the phase III, double-blinded, placebo- and active comparator-controlled VOYAGE 1 trial. J Am Acad Dermatol. 2017;76(3):405–17.
Reich K, Armstrong AW, Foley P, et al. Efficacy and safety of guselkumab, an anti-interleukin-23 monoclonal antibody, compared with adalimumab for the treatment of patients with moderate to severe psoriasis with randomized withdrawal and retreatment: results from the phase III, double-blind, placebo- and active comparator-controlled VOYAGE 2 trial. J Am Acad Dermatol. 2017;76(3):418–31.
Reich K, Gooderham M, Thaçi D, et al. Risankizumab compared with adalimumab in patients with moderate-to-severe plaque psoriasis (IMMvent): a randomised, double-blind, active-comparator-controlled phase 3 trial. Lancet. 2019;394(10198):576–86.
Blauvelt A, Leonardi CL, Gooderham M, et al. Efficacy and safety of continuous risankizumab therapy vs treatment withdrawal in patients with moderate to severe plaque psoriasis: a phase 3 randomized clinical trial. JAMA Dermatol. 2020;156(6):649–58.
Reich K, Papp KA, Blauvelt A, et al. Tildrakizumab versus placebo or etanercept for chronic plaque psoriasis (reSURFACE 1 and reSURFACE 2): results from two randomised controlled, phase 3 trials. Lancet. 2017;390(10091):276–88.
Menter A, Tyring SK, Gordon K, et al. Adalimumab therapy for moderate to severe psoriasis: A randomized, controlled phase III trial. J Am Acad Dermatol. 2008;58(1):106–15.
Saurat JH, Stingl G, Dubertret L, et al. Efficacy and safety results from the randomized controlled comparative study of adalimumab vs. methotrexate vs. placebo in patients with psoriasis (CHAMPION). Br J Dermatol. 2008;158(3):558–66.
M13-606 curated database, adalimumab. Clinicaltrials.gov identification number NCT01646073. Data on file, Eli Lilly and Company.
Lebwohl M, Blauvelt A, Paul C, et al. Certolizumab pegol for the treatment of chronic plaque psoriasis: Results through 48 weeks of a phase 3, multicenter, randomized, double-blind, etanercept- and placebo-controlled study (CIMPACT). J Am Acad Dermatol. 2018;79(2):266-276.e5.
Gottlieb AB, Blauvelt A, Thaci D, et al. Certolizumab pegol for the treatment of chronic plaque psoriasis: Results through 48 weeks from 2 phase 3, multicenter, randomized, double-blinded, placebo-controlled studies (CIMPASI-1 and CIMPASI-2). J Am Acad Dermatol. 2018;79(2):302-314.e6.
Papp KA, Tyring S, Lahfa M, et al. A global phase III randomized controlled trial of etanercept in psoriasis: safety, efficacy, and effect of dose reduction. Br J Dermatol. 2005;152(6):1304–12.
20070559 curated database, etanercept. Clinicaltrials.gov identification number NCT01001208. Data on file, Eli Lilly and Company.
Reich K, Gooderham M, Green L, et al. The efficacy and safety of apremilast, etanercept and placebo in patients with moderate-to-severe plaque psoriasis: 52-week results from a phase IIIb, randomized, placebo-controlled trial (LIBERATE). J Eur Acad Dermatol Venereol. 2017;31(3):507–17.
Reich K, Nestle FO, Papp K, et al. Infliximab induction and maintenance therapy for moderate-to-severe psoriasis: a phase III, multicentre, double-blind trial. Lancet. 2005;366(9494):1367–74.
Menter A, Feldman SR, Weinstein GD, et al. A randomized comparison of continuous vs. intermittent infliximab maintenance regimens over 1 year in the treatment of moderate-to-severe plaque psoriasis. J Am Acad Dermatol. 2007;56(1):31.e1–15.
Cochrane Handbook for Systematic Reviews of Interventions. 25 June 2019 [updated 25 June 2019; cited March 7, 2019]; Available from: http://training.cochrane.org/handbook
Armstrong AW, Betts KA, Signorovitch JE, et al. Number needed to treat and costs per responder among biologic treatments for moderate-to-severe psoriasis: a network meta-analysis. Curr Med Res Opin. 2018;34(7):1325–33.
Núñez M, Huete T, de la Cueva P, et al. A cost-per-number needed to treat analysis assessing the efficiency of biologic drugs in moderate to severe plaque psoriasis. Actas Dermosifiliogr (Engl Ed). 2019;110(7):546–53.
Blauvelt A, Leonardi C, Elewski B, et al. A head-to-head comparison of ixekizumab vs. guselkumab in patients with moderate-to-severe plaque psoriasis: 24-week efficacy and safety results from a randomized, double-blinded trial. Br J Dermatol. 2021;184(6):1047–58.
Rasmussen MK, Enger M, Dahlborn AK, et al. The importance of achieving clear or almost clear skin for patients: results from the nordic countries of the global. Acta Derm Venereol. 2019;99(2):158–63.
Acknowledgements
Funding
Sponsorship for this study and the Rapid Service Fee were funded by Eli Lilly and Company. Richard B. Warren is supported by the Manchester NIHR Biomedical Research Centre. All authors had full access to all of the data in this study and take complete responsibility for the integrity of the data and accuracy of the analysis.
Medical Writing Assistance
Medical writing support was provided by Melody Pupols, PhD (an employee of Syneos Health), and support for this assistance was funded by Eli Lilly and Company.
Authorship
All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this article, take responsibility for the integrity of the work as a whole, and have given their approval for this version to be published.
Authorship Contributions
All authors contributed to the study conception and design, interpretation of data, and provided critical revisions of the manuscript for important intellectual content. All authors read and approved the final manuscript. Material preparation, data collection and analysis were performed by Kyoungah See, Zhuoer Sun, and Ying Zhang.
Prior Presentation
Portions of this work were presented at the Maui Derm Virtual Congress; June 24–27, 2020.
Disclosures
Craig L. Leonardi has served as a consultant/advisory board for AbbVie, Amgen, Boehringer Ingelheim, Dermira, Eli Lilly and Company, Janssen, Leo, Pfizer, Sandoz, UCB, and Vitae; has been an investigator for Actavis, AbbVie, Allergan, Amgen, Boehringer Ingelheim, Celgene, Coherus, Cellceutix, Corrona, Dermira, Eli Lilly and Company, Galderma, Glenmark, Janssen, Leo Pharma, Merck, Novartis, Novella, Pfizer, Sandoz, Sienna, Stiefel, UCB, and Wyeth; and has been a member of the speaker bureau for AbbVie, Amgen, Celgene, Eli Lilly and Company, Janssen, Novartis, Ortho Dermatologics, Sun Pharmaceuticals, and UCB. Kyoungah See, Russel Burge, Ying Zhang, Lotus Mallbris, and Alyssa Garrelts are employees and shareholders of Eli Lilly and Company. Zhuoer Sun is a former employee of Eli Lilly and Company and his current affiliation is with AstraZeneca. Richard B. Warren has been a consultant and/or speaker for AbbVie, Almirall, Amgen, Boehringer Ingelheim, Celgene, Eli Lilly and Company, GlaxoSmithKline, Janssen, Leo Pharma, Novartis, Sanofi Genzyme, and UCB Pharma.
Compliance with Ethics Guidelines
This article is based on previously conducted studies and does not contain any new studies with human participants or animals performed by any of the authors.
Data Availability
The ongoing search is registered at PROSPERO (CRD42021244387).
Author information
Authors and Affiliations
Corresponding author
Supplementary Information
Below is the link to the electronic supplementary material.
Supplementary Video 1: Relative response of biologic treatments of moderate-to-severe plaque psoriasis based on BNMA for PASI 90 (MP4 1593 KB)
Supplementary Video 2: NNT to achieve PASI 90 of biologic treatments of moderate-to-severe plaque psoriasis (MP4 1353 KB)
Supplementary Video 3: Relative response of biologic treatments of moderate-to-severe plaque psoriasis based on Bayesian NMA for PASI 100 (MP4 1676 KB)
Supplementary Video 4: NNT to achieve PASI 100 of biologic treatments of moderate-to-severe plaque psoriasis (MP4 1016 KB)
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Leonardi, C.L., See, K., Burge, R. et al. Number Needed to Treat Network Meta-Analysis to Compare Biologic Drugs for Moderate-to-Severe Psoriasis. Adv Ther 39, 2256–2269 (2022). https://doi.org/10.1007/s12325-022-02065-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12325-022-02065-w