Abstract
Introduction
This post hoc analysis examines the relationship between glycemic variability (GV) and fasting plasma glucose (FPG) targets used to achieve glycated hemoglobin (HbA1c) < 7%, and HbA1c levels after 24 weeks of treatment with insulin glargine and oral antidiabetic drugs (OADs) in Chinese participants with type 2 diabetes mellitus (T2DM) from the BEYOND III FPG GOAL trial (NCT02545842).
Methods
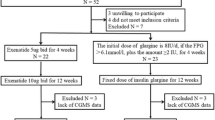
Participants were randomized for three FBG targets (≤ 5.6 mmol/L, ≤ 6.1 mmol/L, and ≤ 7.0 mmol/L) receiving insulin glargine 100 U/mL were analyzed for mean change from baseline to 24 weeks in postprandial glucose (PPG) excursion and FPG coefficient of variation (FPG-CV). The study analyzed change from baseline in HbA1c and the proportion of participants who achieved HbA1c < 7% at 24 weeks, according to their baseline FPG-CV and change from baseline in PPG excursion.
Results
The change in PPG excursion and FPG-CV from baseline to 24 weeks was not significantly different between the three groups stratified by randomization or by 24-week FPG levels. While the change in HbA1c from baseline to 24 weeks was slightly higher among participants with baseline FPG-CV < 33.3% (vs. > 66.7%; P = 0.023), a higher proportion of participants with baseline FPG-CV < 33.3% achieved HbA1c < 7% (P = 0.021).
Conclusions
GV was not associated with either target FPG levels or HbA1c < 7.0% after 24 weeks of treatment with insulin glargine and OADs.
Trial Registration
Clinicaltrials.gov identifier NCT02545842.
Similar content being viewed by others
References
Association AD. 6. Glycemic targets: standards of medical care in diabetes—2020. Diabetes Care. 2020;43:S66-76.
Subramanian S, Hirsch IB. Personalized diabetes management: moving from algorithmic to individualized therapy. Diabetes Spectrum. 2014;27:87–91.
Chon S, Lee YJ, Fraterrigo G, Pozzilli P, Choi MC, Kwon M-K, et al. Evaluation of glycemic variability in well-controlled type 2 diabetes mellitus. Diabetes Technol Ther. 2013;15:455–60.
Saisho Y. Glycemic variability and oxidative stress: a link between diabetes and cardiovascular disease? Int J Mol Sci. 2014;15:18381–406.
Wang P, Huang R, Lu S, Xia W, Sun H, Sun J, et al. HbA1c below 7 % as the goal of glucose control fails to maximize the cardiovascular benefits: a meta-analysis. Cardiovasc Diabetol. 2015;14:124.
Ceriello A, Ihnat MA. “Glycaemic variability”: a new therapeutic challenge in diabetes and the critical care setting. Diabet Med. 2010;27:862–7.
DeVries JH. Glucose variability: where it is important and how to measure it. Diabetes. 2013;62:1405–8.
Xu F, Zhao L-H, Su J-B, Chen T, Wang X-Q, Chen J-F, et al. The relationship between glycemic variability and diabetic peripheral neuropathy in type 2 diabetes with well-controlled HbA1c. Diabetol Metab Syndr. 2014;6:139.
Chehregosha H, Khamseh ME, Malek M, Hosseinpanah F, Ismail-Beigi F. A view beyond HbA1c: role of continuous glucose monitoring. Diabetes Ther. 2019;10:853–63.
Imran SA, Agarwal G, Bajaj HS, Ross S. Targets for glycemic control. Can J Diabetes. 2018;42:S42–6.
Hermann JM, Hammes H-P, Rami-Merhar B, Rosenbauer J, Schütt M, Siegel E, et al. HbA1c variability as an independent risk factor for diabetic retinopathy in Type 1 diabetes: a German/Austrian multicenter analysis on 35,891 patients. PLoS ONE. 2014;9: e91137.
Su G, Mi S, Tao H, Li Z, Yang H, Zheng H, et al. Association of glycemic variability and the presence and severity of coronary artery disease in patients with type 2 diabetes. Cardiovasc Diabetol. 2011;10:19.
Tong L, Chi C, Zhang Z. Association of various glycemic variability indices and vascular outcomes in type-2 diabetes patients: a retrospective study. Medicine (Baltimore). 2018;97: e10860.
Ceriello A, Monnier L, Owens D. Glycaemic variability in diabetes: clinical and therapeutic implications. Lancet Diabetes Endocrinol. 2019;7:221–30.
Yang W, Ma J, Yuan G, Li L, Zhang M, Lu Y, et al. Determining the optimal fasting glucose target for patients with type 2 diabetes: results of the multicentre, open-label, randomized-controlled FPG GOAL trial. Diabetes Obes Metab. 2019;21:1973–7.
Yang W, Yang Z, Zhao J, Lu H, Luo T. Assessment of three fasting plasma glucose targets for insulin glargine-based therapy in people with type 2 diabetes mellitus in China: study protocol for a randomized controlled trial. Trials. 2016;17:470.
De Mattia G, Laurenti O, Moretti A. Comparison of glycaemic control in patients with Type 2 diabetes on basal insulin and fixed combination oral antidiabetic treatment: results of a pilot study. Acta Diabetol. 2009;46:67–73.
Brodows RG, Qu Y, Johns D, Kim D, Holcombe JH. Quantifying the effect of exenatide and insulin glargine on postprandial glucose excursions in patients with type 2 diabetes. Curr Med Res Opin. 2008;24:1395–7.
Hanefeld M, Koehler C, Hoffmann C, Wilhelm K, Kamke W, Gerstein H. Effect of targeting normal fasting glucose levels with basal insulin glargine on glycaemic variability and risk of hypoglycaemia: a randomized, controlled study in patients with early Type 2 diabetes. Diabet Med. 2010;27:175–80.
Association AD. 8. Obesity management for the treatment of type 2 diabetes: standards of medical care in diabetes—2019. Diabetes Care. 2019;42:S81–9.
Home P, Haddad J, Latif ZA, Soewondo P, Benabbas Y, Litwak L, et al. Comparison of national/regional diabetes guidelines for the management of blood glucose control in non-western countries. Diabetes Ther. 2013;4:91–102.
Suh S, Kim JH. Glycemic variability: how do we measure it and why is it important? Diabetes Metab J. 2015;39:273–82.
Zhao Q, Zhou F, Zhang Y, Zhou X, Ying C. Fasting plasma glucose variability levels and risk of adverse outcomes among patients with type 2 diabetes: a systematic review and meta-analysis. Diabetes Res Clin Pract. 2019;148:23–31.
Gimeno-Orna JA, Castro-Alonso FJ, Boned-Juliani B, Lou-Arnal LM. Fasting plasma glucose variability as a risk factor of retinopathy in Type 2 diabetic patients. J Diabetes Complications. 2003;17:78–81.
Sartore G, Chilelli NC, Burlina S, Di Stefano P, Piarulli F, Fedele D, et al. The importance of HbA1c and glucose variability in patients with type 1 and type 2 diabetes: outcome of continuous glucose monitoring (CGM). Acta Diabetol. 2012;49(Suppl 1):S153-160.
Acknowledgements
Funding
This study was funded by Sanofi including the journal’s Rapid Service Fee.
Medical Writing and Editorial Assistance
Editorial assistance in the preparation of this article was provided by Nishad Parkar, PhD, of inScience Communications Springer Healthcare and Anwesha Mandal and Dr G. Kaushik Subramanian of Indegene Pvt. Ltd., Bangalore, India. Support for this medical writing assistance was funded by Sanofi.
Authorship
All named authors met the International Committee of Medical Journal Editors criteria for authorship of this article, take responsibility for the integrity of the work as a whole, and have given their approval for this version to be published.
Authors’ Contributions
The study was designed by Wenying Yang. Ling Li, Tao Yang, Yaoming Xue, and Wenying Yang supervised individual study centers, recruited participants, and conducted the study. Yunguang Li, Xia Zhang, and Nan Cui participated in the study design and coordination between study centers. Juan Du coordinated the manuscript development process, reviewed and proofread the contents, and adjusted the structure. All authors contributed to writing and reviewing the manuscript before submission. Wenying Yang is the guarantor for this manuscript.
Disclosures
Ling Li, Tao Yang, Yaoming Xue, and Wenying Yang have received honoraria for speakers’ bureau and advisory board participation from Sanofi Aventis, Novo Nordisk, AstraZeneca, Bayer, Boehringer Ingelheim, Eli Lilly, Merck Sharp & Dohme, Merck, and Servier. Wenying Yang has also received investigator-initiated trial research grants from AstraZeneca outside of the submitted work. Pengfei Ruan, Juan Du, Yunguang Li, Xia Zhang, and Nan Cui are employees of Sanofi China.
Compliance with Ethics Guidelines
The study was conducted in accordance with the principles stated in the Declaration of Helsinki and in line with the International Council on Harmonisation of Technical Requirements for Pharmaceuticals for Human Use guidelines for good clinical practice. An institutional review board at each site approved the study. All study participants provided written informed consent before study commencement.
Data Availability
Qualified researchers may request access to patient-level data and related study documents including the clinical study report, study protocol with any amendments, blank case report form, statistical analysis plan, and data set specifications. Patient-level data will be anonymized, and study documents will be redacted to protect the privacy of trial participants. Further details on Sanofi’s data sharing criteria, eligible studies, and process for requesting access can be found at https://www.clinicalstudydatarequest.com.
Author information
Authors and Affiliations
Corresponding author
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Li, L., Yang, T., Xue, Y. et al. Influence of Fasting Plasma Glucose Targets on Glycemic Variability in Chinese Participants With Type 2 Diabetes: A Post Hoc Analysis of the FPG GOAL Trial (BEYOND III). Adv Ther 39, 421–429 (2022). https://doi.org/10.1007/s12325-021-01932-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12325-021-01932-2