Abstract
Introduction
Recently the phase 3 BEACON trial showed that the combination of encorafenib, cetuximab, and binimetinib versus cetuximab and irinotecan/FOLFIRI improved overall survival in pre-treated patients with metastatic colorectal cancer (mCRC) with BRAF V600E mutation. However, whether the benefits of these therapies justify their high costs has not been estimated in the USA. The purpose of this study was to evaluate the cost-effectiveness of BEC (binimetinib, encorafenib, and cetuximab), EC (encorafenib and cetuximab), and CI/CF (cetuximab with irinotecan or FOLFIRI) in patients with BRAF V600E-mutated mCRC after first- and second-line therapy.
Methods
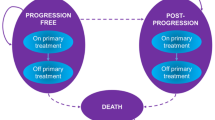
A Markov model was constructed to determine the costs and effects of BEC, EC, and CI/CF on the basis of BEACON trial outcomes data. Health outcomes were measured in life years (LYs), quality-adjusted life years (QALYs), and incremental cost-effectiveness ratios (ICERs). Deterministic and probabilistic sensitivity analyses characterized parameters influencing cost-effectiveness. Subgroup analyses were conducted as well.
Results
The QALYs gained in BEC, EC, and CI/CF were 0.62, 0.54, and 0.40, respectively. BEC resulted in ICERs of $883,895.73/QALY and $1,646,846.14/QALY versus CI/CF and EC, respectively. Compared with CI/CF, the ICER was $435,449.88/QALY in EC. The most sensitive parameters in the comparison among the three arms were the utilities of progressive disease and progression-free survival. Probabilistic sensitivity analyses showed that the probability of BEC and EC being cost-effective was 0%. In subgroup analyses, the ICER remained above the willingness-to-pay threshold of $150,000 per QALY.
Conclusion
BEC and EC were not cost-effective regimens for patients with pre-treated mCRC with BRAF V600E mutation.


Similar content being viewed by others
References
Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68(6):394–424.
Van Cutsem E, Cervantes A, Nordlinger B, Arnold D, Group EGW. Metastatic colorectal cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2014;25(Suppl 3):iii1-9.
Mayer RJ, Van Cutsem E, Falcone A, et al. Randomized trial of TAS-102 for refractory metastatic colorectal cancer. N Engl J Med. 2015;372(20):1909–19.
Tie J, Gibbs P, Lipton L, et al. Optimizing targeted therapeutic development: analysis of a colorectal cancer patient population with the BRAF(V600E) mutation. Int J Cancer. 2011;128(9):2075–84.
Tran B, Kopetz S, Tie J, et al. Impact of BRAF mutation and microsatellite instability on the pattern of metastatic spread and prognosis in metastatic colorectal cancer. Cancer. 2011;117(20):4623–32.
van Geel R, Tabernero J, Elez E, et al. A phase Ib dose-escalation study of encorafenib and cetuximab with or without alpelisib in metastatic BRAF-mutant colorectal cancer. Cancer Discov. 2017;7(6):610–9.
Kopetz S, Desai J, Chan E, et al. Phase II pilot study of vemurafenib in patients with metastatic BRAF-mutated colorectal cancer. J Clin Oncol. 2015;33(34):4032–8.
Corcoran RB, Dias-Santagata D, Bergethon K, Iafrate AJ, Settleman J, Engelman JA. BRAF gene amplification can promote acquired resistance to MEK inhibitors in cancer cells harboring the BRAF V600E mutation. Sci Signal. 2010;3(149):ra84.
Prahallad A, Sun C, Huang S, et al. Unresponsiveness of colon cancer to BRAF(V600E) inhibition through feedback activation of EGFR. Nature. 2012;483(7387):100–3.
Corcoran RB, Andre T, Atreya CE, et al. Combined BRAF, EGFR, and MEK inhibition in patients with BRAF(V600E)-mutant colorectal cancer. Cancer Discov. 2018;8(4):428–43.
Corcoran RB, Atreya CE, Falchook GS, et al. Combined BRAF and MEK inhibition with dabrafenib and trametinib in BRAF V600-mutant colorectal cancer. J Clin Oncol. 2015;33(34):4023–31.
Cunningham D, Humblet Y, Siena S, et al. Cetuximab monotherapy and cetuximab plus irinotecan in irinotecan-refractory metastatic colorectal cancer. N Engl J Med. 2004;351(4):337–45.
Kopetz S, Grothey A, Yaeger R, et al. Encorafenib, binimetinib, and cetuximab in BRAF V600E-mutated colorectal cancer. N Engl J Med. 2019;381(17):1632–43.
Kopetz AGS, Van Cutsem E, Yaeger R, Wasan HS, Yoshino T, Desai J. Encorafenib plus cetuximab with or without binimetinib for BRAF V600E metastatic colorectal cancer: Updated survival results from a randomized, three-arm, phase III study versus choice of either irinotecan or FOLFIRI plus cetuximab (BEACON CRC). In: ASCO Annual Meeting: 2020; 2020.
NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines). Colon cancer: NCCN guidelines. Version 1.2021. https://www.nccn.org/professionals/physician_gls/default.aspx#colon Accessed Dec 2020
Neumann PJ, Cohen JT, Weinstein MC. Updating cost-effectiveness—the curious resilience of the $50,000-per-QALY threshold. N Engl J Med. 2014;371(9):796–7.
Weinstein MC. How much are Americans willing to pay for a quality-adjusted life year? Med Care. 2008;46(4):343–5.
Bertram MY, Lauer JA, De Joncheere K, et al. Cost-effectiveness thresholds: pros and cons. Bull World Health Organ. 2016;94(12):925–30.
Goldstein DA, Chen Q, Ayer T, et al. First- and second-line bevacizumab in addition to chemotherapy for metastatic colorectal cancer: a United States-based cost-effectiveness analysis. J Clin Oncol. 2015;33(10):1112–8.
Cho SK, Hay JW, Barzi A. Cost-effectiveness analysis of regorafenib and TAS-102 in refractory metastatic colorectal cancer in the United States. Clin Colorectal Cancer. 2018;17(4):e751–61.
https://www.drugs.com/price-guide/braftovi. Accessed May 2020.
https://www.drugs.com/price-guide/mektovi. Accessed May 2020.
https://www.drugs.com/price-guide/stivarga. Accessed May 2020.
2020 ASP Drug Pricing Files.2020. https://www.cms.gov/Medicare/Medicare-Fee-forService-Part-B-Drugs/McrPartBDrugAvgSalesPrice/2020ASPFiles.html. Accessed May 2020.
Mistry R, May JR, Suri G, et al. Cost-effectiveness of ribociclib plus letrozole versus palbociclib plus letrozole and letrozole monotherapy in the first-line treatment of postmenopausal women with HR+/HER2− advanced or metastatic breast cancer: a US payer perspective. J Manag Care Spec Pharm. 2018;24(6):514–23.
Perrin A, Sherman S, Pal S, et al. Lifetime cost of everolimus vs axitinib in patients with advanced renal cell carcinoma who failed prior sunitinib therapy in the US. J Med Econ. 2015;18(3):200–9.
Chu JN, Choi J, Ostvar S, et al. Cost-effectiveness of immune checkpoint inhibitors for microsatellite instability-high/mismatch repair-deficient metastatic colorectal cancer. Cancer. 2019;125(2):278–89.
Zhang Y, Baik SH, Fendrick AM, Baicker K. Comparing local and regional variation in health care spending. N Engl J Med. 2012;367(18):1724–31.
Hong DS, Morris VK, El Osta B, et al. Phase IB study of vemurafenib in combination with irinotecan and cetuximab in patients with metastatic colorectal cancer with BRAFV600E mutation. Cancer Discov. 2016;6(12):1352–65.
Yaeger R, Cercek A, O’Reilly EM, et al. Pilot trial of combined BRAF and EGFR inhibition in BRAF-mutant metastatic colorectal cancer patients. Clin Cancer Res. 2015;21(6):1313–20.
Van Cutsem E, Huijberts S, Grothey A, et al. Binimetinib, encorafenib, and cetuximab triplet therapy for patients with BRAF V600E-mutant metastatic colorectal cancer: safety lead-in results from the phase III BEACON colorectal cancer study. J Clin Oncol. 2019;37(17):1460–9.
Prasad V, De Jesus K, Mailankody S. The high price of anticancer drugs: origins, implications, barriers, solutions. Nat Rev Clin Oncol. 2017;14(6):381–90.
Ramsey SD, Bansal A, Fedorenko CR, et al. Financial insolvency as a risk factor for early mortality among patients with cancer. J Clin Oncol. 2016;34(9):980–6.
Acknowledgements
Funding
This manuscript, including the journal’s Rapid Service Fee, was supported by grants from the Hunan Natural Science Foundation of China (No. 2018JJ3852). All authors had full access to all of the data in this study and took complete responsibility for the integrity and accuracy of the data.
Authorship
All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this article, take responsibility for the integrity of the work as a whole, and have given their approval for this version to be published.
Disclosures
Shuosha Li, Huabin Hu, Dong Ding, Youwen Zhu, Jin Huang have nothing to disclose.
Compliance with Ethics Guidelines
This article is based on previously conducted studies and does not contain any human participant or animal study. Ethics committee approval is not required.
Data Availability
All data generated or analyzed during this study are available from the corresponding author on reasonable request.
Author information
Authors and Affiliations
Corresponding author
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Li, S., Hu, H., Ding, D. et al. Cost-Effectiveness Analysis of Encorafenib, Binimetinib, and Cetuximab in BRAF V600E-Mutated Metastatic Colorectal Cancer in the USA. Adv Ther 38, 1650–1659 (2021). https://doi.org/10.1007/s12325-021-01627-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12325-021-01627-8