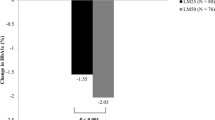
Abstract
Introduction
FPG GOAL was a 24-week, open-label, treat-to-target randomized controlled trial which demonstrated that the optimal self-monitored fasting blood glucose (SM-FBG) target for most Chinese individuals with type 2 diabetes (T2D) using insulin glargine 100 IU/mL was 3.9–6.1 mmol/L. Individuals who achieved lower fasting plasma glucose (FPG) levels might achieve the target HbA1c of < 7% without increasing the risk of hypoglycemia.
Methods
For this post hoc analysis, individuals were redivided into three groups based on their actual laboratory FPG levels at 24 weeks: level 1, ≤ 5.6 mmol/L; level 2, > 5.6 to ≤ 6.1 mmol/L; and level 3, > 6.1 to ≤ 7.0 mmol/L.
Results
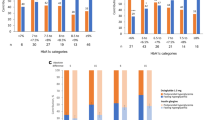
At week 24, 863 individuals with diabetes had available FPG data and 179, 122, and 179 individuals achieved FPG levels 1, 2, and 3, respectively. The proportion of individuals with HbA1c < 7% or HbA1c < 7% without hypoglycemia (≤ 3.9 or ≤ 3.0 mmol/L) was significantly higher in FPG levels 1 (p < 0.01) and 2 (p < 0.05) than in level 3. The least squares mean changes from baseline in HbA1c (− 1.77% and − 1.66% vs − 1.34%; both p < 0.001) and 2-h postprandial glucose (− 3.88 mmol/L and − 3.98 mmol/L vs − 3.22 mmol/L; both p < 0.05) were also significantly higher in FPG levels 1 and 2 compared with level 3. Linear regression analysis showed a moderate relationship between FPG and HbA1c levels at 24 weeks (r = 0.449).
Conclusions
Chinese individuals with T2D who achieved lower FPG levels with insulin glargine 100 IU/mL were more likely to achieve the recommended target HbA1c of < 7% compared with those with higher FPG levels. ClinicalTrials.gov identifier NCT02545842.



Similar content being viewed by others
References
Stratton IM, Adler AI, Neil HAW, et al. Association of glycaemia with macrovascular and microvascular complications of type 2 diabetes (UKPDS 35): prospective observational study. BMJ. 2000;321:405–12.
Yamagishi S, Imaizumi T. Diabetic vascular complications: pathophysiology, biochemical basis and potential therapeutic strategy. Curr Pharm Des. 2005;11:2279–99.
Zoungas S, Chalmers J, Ninomiya T, et al. Association of HbA1c levels with vascular complications and death in patients with type 2 diabetes: evidence of glycaemic thresholds. Diabetologia. 2012;55:636–43.
Riddle M, Umpierrez G, DiGenio A, Zhou R, Rosenstock J. Contributions of basal and postprandial hyperglycemia over a wide range of A1C levels before and after treatment intensification in type 2 diabetes. Diabetes Care. 2011;34:2508–14.
Owens DR, Traylor L, Dain M-P, Landgraf W. Efficacy and safety of basal insulin glargine 12 and 24 weeks after initiation in persons with type 2 diabetes: a pooled analysis of data from treatment arms of 15 treat-to-target randomised controlled trials. Diabetes Res Clin Pract. 2014;106:264–74.
Monnier L, Colette C, Owens D. Postprandial and basal glucose in type 2 diabetes: assessment and respective impacts. Diabetes Technol Ther. 2011;13(Suppl 1):S25–32.
Blonde L, Merilainen M, Karwe V, Raskin P, TITRATE Study Group. Patient-directed titration for achieving glycaemic goals using a once-daily basal insulin analogue: an assessment of two different fasting plasma glucose targets - the TITRATE study. Diabetes Obes Metab. 2009;11:623–31.
Yang W, Ma J, Yuan G, et al. Determining the optimal fasting glucose target for patients with type 2 diabetes: results of the multicentre, open-label, randomized-controlled FPG GOAL trial. Diabetes Obes Metab. 2019;21:1973–7.
Yang W, Yang Z, Zhao J, Lu H, Luo T. Assessment of three fasting plasma glucose targets for insulin glargine-based therapy in people with type 2 diabetes mellitus in China: study protocol for a randomized controlled trial. Trials. 2016;17:470.
Monnier L, Lapinski H, Colette C. Contributions of fasting and postprandial plasma glucose increments to the overall diurnal hyperglycemia of type 2 diabetic patients: variations with increasing levels of HbA1c. Diabetes Care. 2003;26:881–5.
Schernthaner G, Guerci B, Gallwitz B, et al. Impact of postprandial and fasting glucose concentrations on HbA1c in patients with type 2 diabetes. Diabetes Metab. 2010;36:389–94.
Woerle HJ, Neumann C, Zschau S, et al. Impact of fasting and postprandial glycemia on overall glycemic control in type 2 diabetes: importance of postprandial glycemia to achieve target HbA1c levels. Diabetes Res Clin Pract. 2007;77:280–5.
Yki-Järvinen H, Kauppinen-Mäkelin R, Tiikkainen M, et al. Insulin glargine or NPH combined with metformin in type 2 diabetes: the LANMET study. Diabetologia. 2006;49:442–51.
Ramachandran A, Riddle MC, Kabali C, Gerstein HC. Relationship between A1C and fasting plasma glucose in dysglycemia or type 2 diabetes: an analysis of baseline data from the ORIGIN trial. Diabetes Care. 2012;35:749–53.
Guan X, Zheng L, Sun G, et al. The changing relationship between HbA1c and FPG according to different FPG ranges. J Endocrinol Invest. 2016;39:523–8.
Acknowledgements
The authors thank the participants included in the study.
Funding
This study and the journal's rapid service fee were funded by Sanofi.
Authorship
All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this article, take responsibility for the integrity of the work as a whole, and have given their approval for this version to be published.
Authorship Contributions
The study was designed by Wenying Yang, Jianhua Ma, and Minxiang Lei who were in charge of individual study centers, recruited patients, and conducted the study. Yunguang Li, Xia Zhang, and Nan Cui participated in study design and coordinated between study centers. All authors contributed to writing and reviewing the manuscript before submission. Wenying Yang is the guarantor for this manuscript.
Medical Writing and Editorial Assistance
The authors would like to thank Nishad Parkar, PhD, of inScience Communications, Springer Healthcare, for writing the outline and the subsequent drafts of this manuscript. This medical writing assistance was funded by Sanofi.
Disclosures
Wenying Yang has received honoraria for speakers’ bureau and advisory board participation from Sanofi Aventis, Novo Nordisk, AstraZeneca, Bayer, Boehringer Ingelheim, Eli Lily, Merck Sharp & Dohme, Merck, and Servier and has also received investigator-initiated trial research grants from AstraZeneca outside of the submitted work. Jianhua Ma and Minxiang Lei have no conflicts of interest to disclose. Yunguang Li, Xia Zhang, and Nan Cui are employees of Sanofi China.
Compliance with Ethics Guidelines
The study was conducted in accordance with the principles stated in the Declaration of Helsinki and in line with the International Conference on Harmonization Guidelines for Good Clinical Practice. An institutional review board at each site approved the study, and all participants gave written informed consent to participate. The study protocol and the informed consent to participate were reviewed and approved by China-Japan Friendship Hospital Ethics Committee.
Data Availability
Qualified researchers may request access to patient level data and related study documents including the clinical study report, study protocol with any amendments, blank case report form, statistical analysis plan, and dataset specifications. Patient level data will be anonymized and study documents will be redacted to protect the privacy of our trial participants. Further details on Sanofi’s data sharing criteria, eligible studies, and process for requesting access can be found at https://www.clinicalstudydatarequest.com/.
Author information
Authors and Affiliations
Corresponding author
Additional information
Digital Features
To view digital features for this article go to https://doi.org/10.6084/m9.figshare.12464729.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Ma, J., Lei, M., Li, Y. et al. Influence of Fasting Glucose Levels on Achieving Glycemic Target in Individuals with Type 2 Diabetes: a Post Hoc Analysis. Adv Ther 37, 3816–3826 (2020). https://doi.org/10.1007/s12325-020-01410-1
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12325-020-01410-1