Abstract
Introduction
This study characterized the multidose pharmacokinetic (PK) characteristics of posaconazole tablets used as prophylactic antifungal therapy in Chinese patients with acute myelogenous leukemia (AML) at risk for invasive fungal infection (IFI).
Methods
Participants in this open-label, single-arm, phase 1b study received posaconazole 300 mg twice daily on day 1 and then once daily for up to 28 days. In the intensive PK sampling subgroup, posaconazole was administered under fasting conditions on days 1 and 8, and blood samples were regularly collected over 24 h. Trough PK sampling was conducted in all participants on days 1, 2, 3, 8, 14, 21, and 28 without regard for food intake. Population PK characteristics were predicted using PK modeling. Primary endpoints were steady-state average concentration (Cavg) and percentage of participants with steady-state Cavg (predicted and observed) > 500 ng/ml. Treatment safety and efficacy were secondary endpoints.
Results
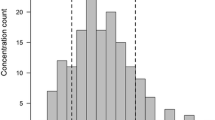
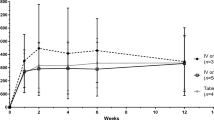
Sixty-five adult Chinese participants were enrolled. On day 8, steady-state arithmetic mean Cavg was 1610 ng/ml (% coefficient of variation [%CV] 42.8%) in the intensive PK subgroup (n = 20). All participants achieved a steady-state Cavg > 500 ng/ml. Predicted Cavg (pCavg) was 1770 ng/ml (%CV 33.7%) in the total population (n = 64); 92.2% of participants had pCavg values ≥ 500 ng/ml (n = 59). The posaconazole tablet safety profile was consistent with that of the oral formulation, and the IFI rate was 3%.
Conclusion
In Chinese AML patients, the posaconazole 300-mg tablet provided PK data comparable with those of previous studies and was generally well tolerated and efficacious.
Clinical Trial Registration
ClinicalTrials.gov, NCT02387983.


Similar content being viewed by others
References
Keating GM. Posaconazole. Drugs. 2005;65(11):1553–677.
Lien MY, Chou CH, Lin CC, et al. Epidemiology and risk factors for invasive fungal infections during induction chemotherapy for newly diagnosed acute myeloid leukemia: a retrospective cohort study. PLoS One. 2018;13(6):e0197851.
Xu XH, Zhang L, Cao XX, et al. Evaluation of the implementation rate of primary antifungal prophylaxis and the prognosis of invasive fungal disease in acute leukemia patients in China. J Infect Chemother. 2017;23(6):360–7.
Sun Y, Huang H, Chen J, et al. Invasive fungal infection in patients receiving chemotherapy for hematological malignancy: a multicenter, prospective, observational study in China. Tumour Biol. 2015;36(2):757–67.
Li Y, Theuretzbacher U, Clancy CJ, Nguyen MH, Derendorf H. Pharmacokinetic/pharmacodynamic profile of posaconazole. Clin Pharmacokinet. 2010;49(6):379–96.
Shen Y, Huang XJ, Wang JX, et al. Posaconazole vs. fluconazole as invasive fungal infection prophylaxis in China: a multicenter, randomized, open-label study. Int J Clin Pharmacol Ther. 2013;51(9):738–45.
Tang L, Yang XF, Qiao M, et al. Posaconazole vs. voriconazole in the prevention of invasive fungal diseases in patients with haematological malignancies: a retrospective study. J Mycol Med. 2018;28(2):379–83.
Wang Y, Xing Y, Chen L, et al. Fluconazole versus mould-active triazoles for primary antifungal prophylaxis in adult patients with acute lymphoblastic leukemia: clinical outcome and cost-effectiveness analysis. Int J Hematol. 2018;107(2):235–43.
Courtney R, Wexler D, Radwanski E, Lim J, Laughlin M. Effect of food on the relative bioavailability of two oral formulations of posaconazole in healthy adults. Br J Clin Pharmacol. 2004;57(2):218–22.
Krishna G, Moton A, Ma L, Medlock MM, McLeod J. Pharmacokinetics and absorption of posaconazole oral suspension under various gastric conditions in healthy volunteers. Antimicrob Agents Chemother. 2009;53(3):958–66.
Pille S, Bohmer D. Options for artificial nutrition of cancer patients. Strahlenther Onkol. 1998;174:52–5.
Vehreschild MJT, Meissner AMK, Cornely OA, et al. Clinically defined chemotherapy-associated bowel syndrome predicts severe complications and death in cancer patients. Haematologica. 2011;96(12):1855–60.
Sansone-Parsons A, Krishna G, Calzetta A, et al. Effect of a nutritional supplement on posaconazole pharmacokinetics following oral administration to healthy volunteers. Antimicrob Agents Chemother. 2006;50(5):1881–3.
Kraft WK, Chang PS, van Iersel MLPS, Waskin H, Krishna G, Kersemaekers WM. Posaconazole tablet pharmacokinetics: lack of effect of concomitant medications altering gastric pH and gastric motility in healthy subjects. Antimicrob Agents Chemother. 2014;58(7):4020–5.
Krishna G, Ma L, Martinho M, O'Mara E. Single-dose phase I study to evaluate the pharmacokinetics of posaconazole new tablet and capsule formulations relative to oral suspension. Antimicrob Agents Chemother. 2012;56(8):4196–201.
Krishna G, Ma L, Martinho M, Preston RA, O'Mara E. A new solid oral tablet formulation of posaconazole: a randomized clinical trial to investigate rising single- and multiple-dose pharmacokinetics and safety in healthy volunteers. J Antimicrob Chemother. 2012;67(11):2725–30.
Duarte RF, López-Jiménez J, Cornely OA, et al. Phase 1b study of new posaconazole tablet for prevention of invasive fungal infections in high-risk patients with neutropenia. Antimicrob Agents Chemother. 2014;58(10):5758–65.
Cornely OA, Duarte RF, Haider S, et al. Phase 3 pharmacokinetics and safety study of a posaconazole tablet formulation in patients at risk for invasive fungal disease. J Antimicrob Chemother. 2016;71(3):718–26.
Cornely OA, Maertens J, Winston DJ, et al. Posaconazole vs fluconazole or itraconazole prophylaxis in patients with neutropenia. N Engl J Med. 2007;356(4):348–59.
Ullmann AJ, Lipton JH, Vesole DH, et al. Posaconazole or fluconazole for prophylaxis in severe graft-versus-host disease. N Engl J Med. 2007;356(4):335–47.
Walsh TJ, Raad I, Patterson TF, et al. Treatment of invasive aspergillosis with posaconazole in patients who are refractory to or intolerant of conventional therapy: an externally controlled trial. Clin Infect Dis. 2007;44(1):2–12.
Shen JX, Krishna G, Hayes RN. A sensitive liquid chromatography and mass spectrometry method for the determination of posaconazole in human plasma. J Pharmaceut Biomed Anal. 2007;43(11):228–36.
de Pauw B, Walsh TJ, Donnelly JP, et al. Revised definitions of invasive fungal disease from the European Organization for Research and Treatment of Cancer/Invasive Fungal Infections Cooperative Group and the National Institute of Allergy and Infectious Diseases Mycoses Study Group (EORTC/MSG) Consensus Group. Clin Infect Dis. 2008;46:1813–21.
van Iersel MLPS, Rossenu S, de Greef R, Waskin H. A population pharmacokinetic model for a solid oral tablet formulation of posaconazole. Antimicrob Agents Chemother. 2018;62(7):e02465–e2517.
Li H, Wei Y, Zhang S, et al. Pharmacokinetics and safety of posaconazole administered by intravenous solution and oral tablet in healthy Chinese subjects and effect of food on tablet bioavailability. Clin Drug Investig. 2019;39(11):1109–16.
Jung DS, Tverdek FP, Kontoyiannis DP. Switching from posaconazole suspension to tablets increases serum drug levels in leukemia patients without clinically relevant hepatotoxicity. Antimicrob Agents Chemother. 2014;58(11):6993–5.
Cumpston A, Caddell R, Shillingburg A, et al. Superior serum concentrations with posaconazole delayed-release tablets compared to suspension formulation in hematological malignancies. Antimicrob Agents Chemother. 2015;59(8):4424–8.
Belling M, Kanate AS, Shillingburg A, et al. Evaluation of serum posaconazole concentrations in patients with hematological malignancies receiving posaconazole suspension compared to the delayed-release tablet formulation. Leuk Res Treatment. 2017;2017:3460892.
Durani U, Tosh PK, Barreto JN, et al. Retrospective comparison of posaconazole levels in patients taking the delayed-release tablet versus the oral suspension. Antimicrob Agents Chemother. 2015;59(8):4914–8.
Leclerc E, Combarel D, Uzunov M, et al. Prevention of invasive Aspergillus fungal infections with the suspension and delayed-release tablet formulations of posaconazole in patients with haematologic malignancies. Sci Rep. 2018;8(1):1681.
Pham AN, Bubalo JS, Lewis JS 2nd. Comparison of posaconazole serum concentrations from haematological cancer patients on posaconazole tablet and oral suspension for treatment and prevention of invasive fungal infections. Mycoses. 2016;59(4):226–33.
Liebenstein TK, Widmer KM, Fallon MJ. Retrospective analysis of goal drug level attainment of posaconazole for invasive fungal infection prophylaxis in patients with acute myeloid leukemia pre- and post-switch to tablet formulation. J Oncol Pharm Pract. 2018;24(8):599–603.
Lenczuk D, Zinke-Cerwenka W, Greinix H, et al. Antifungal prophylaxis with posaconazole delayed-release tablet and oral suspension in a real-life setting: plasma levels, efficacy, and tolerability. Antimicrob Agents Chemother. 2018;62:6.
Suh HJ, Kim I, Cho JY, et al. Comparison of plasma concentrations of posaconazole with the oral suspension and tablet in Korean patients with hematologic malignancies. Infect Chemother. 2017;49(2):135–9.
Furuno JP, Tallman GB, Noble BN, et al. Clinical outcomes of oral suspension versus delayed-release tablet formulations of posaconazole for prophylaxis of invasive fungal infections. Antimicrob Agents Chemother. 2018;62:10.
Boglione-Kerrien C, Picard S, Tron C, et al. Safety study and therapeutic drug monitoring of the oral tablet formulation of posaconazole in patients with haematological malignancies. J Cancer Res Clin Oncol. 2018;144(1):127–34.
Martson AG, Veringa A, van den Heuvel ER, et al. Posaconazole therapeutic drug monitoring in clinical practice and longitudinal analysis of the effect of routine laboratory measurements on posaconazole concentrations. Mycoses. 2019;62(8):698–705.
Kosmidis C, Rodriguez-Goncer I, Rautemaa-Richardson R, et al. Therapeutic drug monitoring and adverse events of delayed-release posaconazole tablets in patients with chronic pulmonary aspergillosis. J Antimicrob Chemother. 2019;74(4):1056–61.
Petitcollin A, Boglione-Kerrien C, Tron C, et al. Population pharmacokinetics of posaconazole tablets and Monte Carlo simulations to determine whether all patients should receive the same dose. Antimicrob Agents Chemother. 2017;61:11.
Peterlin P, Chauvin C, Le Gouill S, et al. Fungal prophylaxis with a gastro-resistant posaconazole tablet for patients with hematological malignancies in the POSANANTES study. Antimicrob Agents Chemother. 2018;62:2.
Lewis RE, Kontoyiannis DP, Viale P, Sarpong EM. Using state transition models to explore how the prevalence of subtherapeutic posaconazole exposures impacts the clinical utility of therapeutic drug monitoring for posaconazole tablets and oral suspension. Antimicrob Agents Chemother. 2019;63:12.
Pettit NN, Miceli MH, Rivera CG, et al. Multicentre study of posaconazole delayed-release tablet serum level and association with hepatotoxicity and QTc prolongation. J Antimicrob Chemother. 2017;72(8):2355–8.
Kersemaekers WM, Dogterom P, Xu J, et al. Effect of a high-fat meal on the pharmacokinetics of 300-milligram posaconazole in a solid oral tablet formulation. Antimicrob Agents Chemother. 2015;59(6):3385–9.
Witticke D, Seidling HM, Klimm HD, Haefeli WE. Do we prescribe what patients prefer? Pilot study to assess patient preferences for medication regimen characteristics. Patient Prefer Adherence. 2012;6:679–84.
Cojutti PG, Candoni A, Lazzarotto D, et al. Co-administration of proton pump inhibitors and/or of steroids may be a risk factor for low trough concentrations of posaconazole delayed-released tablets in adult patients with haematological malignancies. Br J Clin Pharmacol. 2018;84(11):2544–50.
Phillips K, Cirrone F, Ahuja T, Siegfried J, Papadopoulos J. Posaconazole versus voriconazole as antifungal prophylaxis during induction therapy for acute myelogenous leukemia or myelodysplastic syndrome. J Oncol Pharm Pract. 2019;25(2):398–403.
Coleman CI, Limone B, Sobieraj DM, et al. Dosing frequency and medication adherence in chronic disease. J Manag Care Pharm. 2012;18(7):527–39.
Chen L, Wang Y, Zhang T, et al. Utility of posaconazole therapeutic drug monitoring and assessment of plasma concentration threshold for effective prophylaxis of invasive fungal infections: a meta-analysis with trial sequential analysis. BMC Infect Dis. 2018;18(1):155.
Acknowledgements
The authors thank the participants of the study.
Funding
The design, study conduct, and financial support for this research were provided by Merck Sharp & Dohme Corp., a subsidiary of Merck & Co., Inc., Kenilworth, NJ, USA. They have also funded the journals Rapid Service fee associated with manuscript publication.
Medical Writing and Editorial Assistance
Medical writing assistance, supported by Merck Sharp & Dohme Corp., a subsidiary of Merck & Co., Inc., Kenilworth, NJ, USA, was provided by Jennifer M. Kulak, PhD, of ApotheCom (Yardley, PA) in the preparation of this article.
Authorship
All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this article, take responsibility for the integrity of the work as a whole, and have given their approval for this version to be published.
Authorship Contributions
KL, DW, JL, HC, TZ, HD, LC, and XMZ contributed to acquisition of the data and reviewing/revising the manuscript. HN contributed to acquisition of the data, interpretation of the results, and reviewing/revising the manuscript. EM contributed to analysis of the data and reviewing/revising the manuscript. GAW and HW contributed to study design, analysis of data, interpretation of results, and reviewing/revising the manuscript. JJ contributed to interpretation of results and reviewing/revising the manuscript. YQ contributed to analysis of the data. All authors reviewed the final version of the manuscript and agreed with its submission.
Disclosures
Kaiyan Liu, Depei Wu, Junmin Li, Hu Chen, Hongmei Ning, Ting Zhao, Haiping Dai, and Li Chen have no conflicts to disclose. Eric Mangin, Hetty Waskin, and Xu Min Zhao are employees of Merck Sharp & Dohme Corp., a subsidiary of Merck & Co., Inc., Kenilworth, NJ, USA (MSD). Gregory A. Winchell is a subcontractor to Certara providing paid services to MSD. Jun Jiang and Yanping Qiu were employees of MSD at the time of the study. Hetty Waskin holds stock in Merck & Co., Inc., Kenilworth, NJ, USA, and is an author on a submitted patent application. Xumin Zhao has changed affiliation from Merck & Co., Inc., Kenilworth, NJ, USA, to Bayer Healthcare Company Limited.
Compliance with Ethics Guidelines
The study was conducted in accordance with the principles of Good Clinical Practice, and written informed consent was obtained from each participant. The protocol was reviewed and approved by independent ethics committees at all participating study centers (Peking University People’s Hospital, The First Affiliated Hospital of Soochow University, The 307th Hospital of Chinese People’s Liberation Army, and Shanghai Ruijin Hospital). This study was performed in accordance with the Helsinki Declaration of 1964 and its later amendments. Informed consent was obtained from all participants included in the study.
Data Availability
The data sets generated and/or analyzed during the current study are not publicly available but are available from http://engagezone.merck.com/ds_documentation.php. Requests for access to the clinical study data can be submitted through the EngageZone site or via email to dataaccess@merck.com.
Author information
Authors and Affiliations
Corresponding author
Additional information
Digital Features
To view digital features for this article go to https://doi.org/10.6084/m9.figshare.12086820.
Rights and permissions
About this article
Cite this article
Liu, K., Wu, D., Li, J. et al. Pharmacokinetics and Safety of Posaconazole Tablet Formulation in Chinese Participants at High Risk for Invasive Fungal Infection. Adv Ther 37, 2493–2506 (2020). https://doi.org/10.1007/s12325-020-01341-x
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12325-020-01341-x