Abstract
Introduction
Reflux symptoms are frequently associated with esophageal acid exposure. However, other potential causes unrelated to acid secretion are possible, and the relationship between acid control and symptomatic improvement remains unclear. This study investigated the correlation between individual intragastric pH control and heartburn relief among subjects with frequent heartburn who are likely to self-treat with over-the-counter (OTC) medications. We hypothesized that improved acid control would provide greater symptomatic improvement among individuals representative of an OTC population.
Methods
This phase 4, single-center, randomized, double-blind, placebo-controlled study was conducted in subjects without diagnosed gastroesophageal reflux disease or other gastrointestinal conditions who were experiencing frequent heartburn (≥ 3 episodes/week; ≥ 2 nighttime episodes/week over past 30 days) that was responsive to treatment. Subjects entered a 7-day run-in phase, received placebo BID (before breakfast and dinner), and completed symptom diaries. During the treatment phase, subjects received esomeprazole 20 mg BID, esomeprazole 20 mg then placebo, or placebo BID. Subjects underwent 24-h intragastric pH monitoring at baseline and day 14 and completed daily symptom diaries.
Results
In the per-protocol population (n = 39), mean (SD) change from baseline in percentage of time with intragastric pH > 4 was 58.7% (± 26.4%) versus 41.0% (± 30.4%) for those who did and did not achieve 24-h heartburn relief. Significant correlations were observed between change in percentage of time with intragastric pH > 4 and 24-h heartburn relief (OR 1.028; 95% CI 1.001, 1.055; P = 0.0442) and complete resolution (OR 1.034; 95% CI 1.003, 1.065; P = 0.0301).
Conclusions
Individuals with greater improvements in duration of intragastric acid suppression had an increased likelihood of achieving heartburn relief and resolution. These results indicate that individuals not adequately controlling their intragastric pH may require an escalation in dose of their acid-suppressive therapy, assessment with 24-h pH monitoring, or a change in treatment regimen to address non-reflux-related etiologies of their heartburn.
Trial Registration
ClinicalTrials.gov identifier: NCT02708355.
Funding
Pfizer Consumer Healthcare, Madison, NJ, USA.
Plain Language Summary
Plain language summary available for this article.
Similar content being viewed by others
Plain Language Summary
In this study, subjects who did not have a diagnosis of gastroesophageal reflux disease, or GERD, but who were experiencing frequent symptomatic heartburn with no diagnostic medical evaluation were randomized to receive either placebo or esomeprazole 20 mg once or twice a day. During the 14-day treatment period, subjects received either esomeprazole 20 mg twice a day, esomeprazole 20 mg and then placebo, or placebo twice a day and completed daily symptom diaries. Subjects’ 24-h intragastric pH was monitored at baseline and on day 14 of the treatment period, using the standard definition of gastric acidity (i.e., pH > 4). The relationship between the change in the percentage of time from baseline to day 14 with 24-h pH > 4, heartburn relief, and complete heartburn resolution was evaluated.
Significant associations were observed between the changes from baseline to day 14 in the individuals’ percentage of time with intragastric pH > 4, 24-h heartburn relief, and complete heartburn resolution.
We concluded that the likelihood of an individual experiencing heartburn relief or resolution improved as the control over intragastric pH increased. These effects have only previously been observed using population-level means, so this is the first time this relationship has been observed using individual data. As expected, esomeprazole treatment increased the likelihood of experiencing treatment response, but symptomatic response was also observed with placebo and was dependent on the individual degree of pH control. These results suggest that individualized assessments can provide guidance in making treatment decisions for individuals not achieving adequate pH control.
Introduction
The role of esophageal acid exposure in reflux symptoms (i.e., heartburn, acid regurgitation) is unequivocal and provides the rationale for using acid-suppressive therapy [1]. In fact, the American College of Gastroenterology recommends empirical use of acid-suppressive therapy to manage reflux symptoms prior to conducting diagnostic investigations [2]. The degree of mucosal injury in erosive esophagitis (EE) is correlated with the amount of time with intragastric pH < 4 [3]. As a result, the percentage of time in a 24-h period when intragastric pH is > 4 is an important metric for assessing the pharmacodynamic effects of acid-suppressive therapies [4,5,6,7,8].
Acid-suppressive therapies can significantly increase the percentage of time with intragastric pH > 4 within 7 days of initiation (P < 0.01) [4, 5]. Sustained suppression of gastric acid secretion with proton-pump inhibitors (PPI) or histamine receptor 2 antagonists (H2RA) plays a role in treating acid-related diseases [1, 9]. PPIs are commonly used in both over-the-counter (OTC) and prescription settings and block the final step of gastric acid production, inhibiting both basal and stimulated acid secretion [1, 10]. In one study, healing rates in EE were positively correlated with percentage of time with intragastric pH > 4 following 4 weeks of esomeprazole treatment [11]. This study also suggested that greater intragastric acid control lowered daytime and nighttime heartburn and acid regurgitation [11]. Extrapolation of the relationship between acid suppression and heartburn relief in individuals with non-erosive reflux is accepted; however, the relationship between the degree of acid suppression and symptom relief on an individual level has not been investigated.
The current pilot proof-of-concept study was designed to explore the relationship between intragastric acid control and reflux symptoms by investigating associations between change from baseline in time with intragastric pH > 4 and 24-h heartburn relief using individual-subject data. We hypothesized that individuals with frequent heartburn would experience greater symptomatic improvement with improved acid control (i.e., greater reductions in pH). Because responses to medicine vary from person to person, individual titration by the consumer may be necessary to optimize outcomes among those who do not achieve complete resolution of heartburn with PPI treatment. Thus, exploring these issues in the context of individuals who may self-treat their reflux symptoms using an OTC PPI will provide additional insights that may potentially guide alternative treatment options for non-responders.
Methods
Study Design
This phase 4, single-center, randomized, double-blind, placebo-controlled, parallel-group pilot study (conducted between January 22, 2016, and April 3, 2016) included a 1-week single-blind placebo run-in phase followed by randomization into a 2-week double-blind treatment phase (Fig. 1). The run-in phase (days − 8 to − 1) was used to wash out any previous H2RA and/or PPI therapy, confirm the incidence of symptoms reported at screening, and determine whether subjects could accurately complete daily diaries. Subjects completed a daily diary to document occurrences of heartburn, acid regurgitation, and epigastric pain during the previous 24-h period. The scale evaluated the presence and absence of symptoms and severity (0 = none; 2 = severe) but not the number of events. Subjects began taking placebo twice daily (before breakfast and dinner) on day − 8 and began reporting symptoms on day − 7 to − 1. On day − 1, subjects also completed the Quality of Life in Reflux and Dyspepsia (QOLRAD) questionnaire.
Following the run-in, subjects returned for potential baseline pH monitoring. Eligible subjects were required to report taking ≥ 80% of placebo doses (≤ 2 missed doses) and experiencing ≥ 1 heartburn episode during three separate 24-h periods; two episodes were required to occur during the night (i.e., nighttime heartburn; occurring during the time after consuming the evening meal until getting up in the morning to start daily activities) in two separate 24-h periods. Eligible subjects were required to be compliant in reporting symptoms via a daily diary for ≥ 6 days of the 7-day run-in. Subjects who met randomization criteria initiated 24-h catheter-based intragastric and esophageal pH monitoring. Subjects remained at the center for ~ 11 h then returned the next day (day 1) to receive their first dose of study medication.
Treatment assignment was determined by a computer-generated randomization schedule generated and maintained by Pfizer Global Randomization Operations, and third-party personnel dispensed study medication. Only these individuals had access to the randomization schedule and dispensing records.
This study complied with the ethical principles of the Declaration of Helsinki and all International Conference for Harmonisation Good Clinical Practice Guidelines. The final protocol, any amendments, and informed consent forms were reviewed and approved on December 9, 2015, by an independent institutional review board at the investigational center (Oklahoma Foundation for Digestive Research; Oklahoma City, OK, USA). Informed consent was obtained from all individual participants included in the study. This study is registered with ClinicalTrials.gov (NCT02708355).
Eligible subjects were randomized to one of three 14-day treatment arms (2:2:1 ratio): esomeprazole 20 mg [administered as 22.3 mg esomeprazole magnesium trihydrate (Nexium® 24HR; Pfizer Consumer Healthcare, Madison, NJ, USA)] before both breakfast and dinner; esomeprazole 20 mg before breakfast/placebo before dinner; and placebo/placebo (both before breakfast and dinner). For non-pH-monitoring days, subjects were instructed to take the first and second daily doses 30–60 min before breakfast and dinner, respectively, to reflect product labeling for OTC PPIs that are used to self-treat heartburn and allow for variability in meal timing when subjects were away from the study center. When possible, breakfast and dinner were separated by ≥ 10 h (e.g., breakfast, 8:00 AM; dinner, 6:00 PM). A chewable antacid containing aluminum hydroxide 200 mg, magnesium hydroxide 200 mg, and simethicone 25 mg (Mintox™ Plus; Major Pharmaceuticals, Livonia, MI, USA) was provided as rescue medication. Subjects chewed one tablet as needed and repeated hourly if symptoms returned (≤ 6 tablets/24-h period and ≤ 28 tablets/7-day period). No rescue medications were allowed during the 24-h pH monitoring period on day − 1 or 14.
Subject diaries recorded the incidence of daily heartburn, acid regurgitation, and epigastric pain in the morning for the previous 24-h period throughout the 14-day treatment period. QOLRAD assessments, which described subjects’ experiences over the previous 7 days, were conducted on day 14. After completing day-14 24-h pH monitoring, subjects returned to the center for final assessments and catheter removal.
Study Population
Subjects were recruited from the study site’s database and through advertising. Inclusion criteria included male and nonpregnant, nonlactating female subjects, 22–65 years of age, with 17.5–45.0 kg/m2 body mass index (BMI) and total body weight > 50 kg (110 lbs), heartburn averaging ≥ 3 episodes/week, including ≥ 2 episodes/week of nighttime heartburn, over the past 30 days, and confirmed heartburn, acid regurgitation, or epigastric pain histories for ≥ 3 months that responded to antacids, H2RAs, and/or PPIs. To reflect a typical population that would self-treat their heartburn with an OTC product, subjects did not undergo diagnostic testing and those with evidence/history of clinically significant diseases including gastrointestinal conditions (other than frequent heartburn), histories of endoscopically verified EE (GERD), and need for continuous H2RAs, PPIs, gastric prokinetic drugs, or antacids for any indication (e.g., subjects with long-term prescription therapy) were not enrolled. The use of empiric PPI therapy to initially treat heartburn is recommended and common clinical practice [2]. Male and female subjects of childbearing potential were required to use highly effective contraceptives throughout the study and for ≥ 28 days after the last treatment dose. Women of nonchildbearing potential must have met ≥ 1 of the following criteria: confirmed postmenopausal status, documented hysterectomy and/or bilateral oophorectomy, or medically confirmed ovarian failure.
Intragastric and Esophageal pH Monitoring
To monitor intragastric and esophageal pH, a catheter probe (ComforTec Z/pH Probe; Sandhill Scientific Inc, Highlands Ranch, CO, USA) was inserted transnasally. A distal esophageal sensor was placed ~ 5 cm above the manometrically located proximal border of the lower esophageal sphincter; another was placed intragastrically ~ 10 cm below the lower esophageal sphincter. The percentage of time with intragastric pH > 4 and esophageal pH < 4 and median intragastric and esophageal pH over 24 h on days − 1 and 14 were evaluated. On pH monitoring days, standardized meals were provided to limit the effects of food choices. Breakfast was served 60 min after starting pH recording of day − 1, and, on day 14, breakfast was served 30 min after morning dosing. On day 14, lunch, snack, and dinner were served at 4, 7, and 10 h, respectively, after morning dosing; evening doses were administered 9.5 h after morning dosing. After dinner, subjects were discharged with the nasal catheter in place and returned home with the pH monitoring data logger, a daily symptom diary, and rescue medication.
Efficacy Evaluations
The primary evaluation was the association between change in percentage of a 24-h day with intragastric pH > 4 and 24-h heartburn relief. Relief of 24-h heartburn was defined as a daily diary response of “0” to the question, “Over the last 24 h (yesterday/last night), what was the severity of your most intense episode of heartburn?” on ≥ 6 of subjects’ last 7 consecutive treatment days, allowing for 1 day with a maximum severity of “2.” All efficacy parameters are described in Table 1.
In addition to objective parameters, the impact on quality of life was evaluated using the QOLRAD [12], which measures the impact on emotional distress, sleep disturbance, food and drink problems, physical and social functioning, and vitality on a 7-point Likert scale. Lower scores indicate more severe impact on daily functioning. Assessments were conducted on days − 1 and 14 and focused on the previous 7 days. Mean scores on each domain were used to calculate improvement from days − 1 to 14.
Safety
The investigator recorded any observed or volunteered adverse events (AEs), including severity (mild, moderate, or severe) and investigator’s opinion of the relationship to treatment. The investigator recorded clinically significant changes in physical examination and abnormal objective test findings (e.g., electrocardiogram, laboratory) as AEs.
Statistics
The per-protocol (PP) analysis set was the primary population of interest and included only evaluable subjects, defined as those who completed the 14-day treatment phase, provided valid data for day 14 pH monitoring, and completed ≥ 5 days of diary entries for days − 7 to − 1 and 8–14. All analyses except for safety assessments were conducted with this population. To further explore the relationship between change from baseline in percentage of time with intragastric pH > 4 and heartburn relief and complete resolution, the full analysis set (FAS) was also analyzed. The FAS population was defined as all randomized subjects who provided valid day − 1 pH monitoring data and took ≥ 1 dose of randomized study medication. Approximately 100 subjects were planned to be enrolled and approximately 75 subjects to be randomized into the treatment phase, so that 30 subjects would be randomized to each esomeprazole 20 mg group and 15 subjects to placebo, with the goal of having ~ 50 subjects complete the study. Owing to the preliminary nature of this study, the sample size was not based on statistical calculations.
Logistic regression analyses using the PP population were performed with relief and complete resolution of 24-h and nighttime heartburn (yes/no) on day 14 (dependent variables) and change in percentage of time with intragastric pH > 4 (independent variable), controlling for age, sex, and BMI. The analyses conducted with the FAS utilized a similar model, controlling for age, sex, and BMI; treatment was also included in this model. Summary measures included odds ratio (OR) for change in percentage of time with intragastric pH > 4 or change in median intragastric pH with 95% confidence interval (CI) of the OR. Boxplots of change in percentage of time with intragastric pH > 4 and change in median intragastric pH were constructed for subjects by status of relief and complete resolution of 24-h and nighttime heartburn.
Linear regressions were performed with improvement in the frequency and severity of 24-h and nighttime heartburn and average daily number of heartburn events at day 14 (dependent variables) and change in percentage of time with intragastric pH > 4 (independent variable), controlling for age, sex, and BMI. Similar models were used with change in median intragastric pH as the independent variable. The coefficient of change in percentage of time with intragastric pH > 4 or change in median intragastric pH was calculated along with associated P values and 95% CIs. SAS® software (version 9.4; SAS Institute; Cary, NC, USA) was used to perform all statistical analyses.
Results
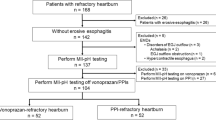
A total of 55 subjects were randomized to one of the three treatment arms; all 55 subjects were included in the FAS population (Fig. 2). The PP population included 39 subjects (16 subjects randomized to esomeprazole 20 mg/esomeprazole 20 mg, 18 subjects randomized to esomeprazole 20 mg/placebo, and 5 subjects randomized to placebo/placebo). Among those excluded from the PP population, most were not evaluable because they took rescue medication for > 2 days during days 8–14 and thus > 2 days of diary data were set to be missing during that period, violating the PP population’s definition. Owing to the nature of this proof-of-concept study, the PP population was used for all endpoint analyses; only select analyses were performed on the FAS population. Results of some FAS analyses, which included 12 placebo subjects, are also reported. Demographic and baseline characteristics of the study population were similar among the three groups (Table 2).
Subject disposition. The per-protocol population included only evaluable subjects, defined as subjects who completed the 14-day treatment phase, underwent and provided valid data for day-14 pH monitoring and completed ≥ 5 days of diary entries on each of days − 7 to − 1 and days 8–14. ESO esomeprazole, FAS full analysis set, PBO placebo, PP per-protocol
Relationship Between Intragastric pH and 24-h Heartburn Relief
In the PP population (n = 39), the mean (± standard deviation [SD]) change from baseline in percentage of time with intragastric pH > 4 was 48.7% (± 29.7%). For the different subgroups, the mean change from baseline was 70.2% (± 23.1%) for esomeprazole 20 mg/esomeprazole 20 mg, 42.8% (± 18.7%) for esomeprazole 20 mg/placebo, and 0.78% (± 5.8%) for placebo/placebo. The mean (SD) change from baseline in percentage of time with intragastric pH > 4 for the portion of the PP population who achieved 24-h heartburn relief was 58.7% (± 26.4%) versus 41.0% (± 30.4%) for those who did not achieve 24-h heartburn relief.
A total of 17 (43.6%) subjects in the PP population reported 24-h heartburn relief, including 7 (43.8%) for esomeprazole 20 mg/esomeprazole 20 mg, 10 (55.6%) for esomeprazole 20 mg/placebo, and 0 (0.0%) for placebo/placebo. The remaining 22 (56.4%) subjects did not report 24-h heartburn relief. For subjects reporting 24-h heartburn relief, the mean (± SD) change from baseline in percentage of time with intragastric pH > 4 was 78.6% (± 14.6%) and 44.7% (± 23.9%) for esomeprazole 20 mg/esomeprazole 20 mg and esomeprazole 20 mg/placebo, respectively.
There was a significant correlation between change in percentage of time with intragastric pH > 4 and 24-h heartburn relief in the PP population, controlling for age, sex, and BMI [OR (95% CI) 1.028 (1.001, 1.055); P = 0.0442; Fig. 3]. A significant relationship was also observed between change in median intragastric pH and 24-h heartburn relief [OR (95% CI) 1.629 (1.002, 2.650); P = 0.0493]. For the FAS population (n = 55), the OR for the relationship between 24-h heartburn relief and change from baseline in the percentage of time with intragastric pH > 4 [OR 1.027 (95% CI 0.991, 1.065)] was directionally similar to the OR in the PP analyses but no longer significant (P = 0.1451). These analyses were adjusted for treatment in the model, which may have led to the loss of statistical significance compared with the PP analyses.
Boxplot of change in percentage of time with intragastric pH > 4 for subjects with relief of 24-h heartburn vs. no relief of 24-h heartburn at day 14, per-protocol population. Thirty-nine subjects are included in this plot for 24-h heartburn at day 14, among whom 17 had relief and 22 did not have relief. This boxplot provides median and the 25th/75th percentiles, with whisker tops at the 90th percentile and whisker bottoms at the 10th percentile. Data points that are outside the percentile range are represented with red squares
Relationship Between Intragastric pH and Other Efficacy Parameters
Complete 24-h heartburn resolution was significantly related to change in percentage of time with intragastric pH > 4, but not with change in median intragastric pH (Table 3; Fig. 4). Similar to the FAS analyses for heartburn relief, the association between complete 24-h heartburn resolution and change in percentage of time with intragastric pH > 4 using the FAS population revealed a trend that was similar to PP analyses but nonsignificant [OR 1.043 (95% CI 0.996, 1.091); P = 0.0737]. Using PP population data, there were also significant associations (P < 0.05) between both change in percentage of time with intragastric pH > 4 and median change in intragastric pH and observed improvement in 24-h heartburn severity, improvement in frequency and severity of nighttime heartburn, and change in percentage of 24-h days without epigastric pain (Table 3).
Boxplot of change in percentage of time with intragastric pH > 4 for subjects with complete resolution of 24-h heartburn vs. no resolution of 24-h heartburn at day 14, per-protocol population. Thirty-nine subjects are included in this plot for 24-h heartburn at day 14, including 13 with complete resolution and 26 without complete resolution. Boxplot provides median and the 25th/75th percentiles, with whisker tops at the 90th percentile and whisker bottoms at the 10th percentile. Data points that are outside the percentile range are represented with red squares
Relationship Between Intragastric pH and QOLRAD
After controlling for age, sex, BMI, and corresponding baseline score, significant correlations were observed between change in percentage of time with intragastric pH > 4 and improvement in the QOLRAD domain scores for emotional distress (P = 0.0381), food/drink problems (P = 0.0476), and physical/social functioning (P = 0.0222). There was no significant association between the percentage of time with intragastric pH > 4 and other QOLRAD domains. Physical/social functioning was the only parameter significantly associated with the change in median intragastric pH (Table 4).
Relationship Between Change in Percentage of Time with Esophageal pH < 4 and Symptom Response
The change in percentage of time with esophageal pH < 4 was significantly correlated with improvements in average daily number of heartburn events (coefficient 1.191; 95% CI 0.688, 1.693; P < 0.0001), 24-h heartburn severity (coefficient 0.120; 95% CI 0.029, 0.211; P = 0.0115), and nighttime heartburn severity (coefficient 0.120; 95% CI 0.036, 0.205; P = 0.0063). There were also significant correlations between change in percentage of time with esophageal pH < 4 and QOLRAD food/drink problems (coefficient 0.478; 95% CI 0.031, 0.924; P = 0.0367) and vitality (coefficient 0.212; 95% CI 0.018, 0.407; P = 0.0333).
Relationship Between Change in Median Esophageal pH and Symptom Response
Overall, the mean (SD) change in percentage of time with esophageal pH < 4 was 4.6% (± 5.8%). The only outcome correlated with change in median esophageal pH was improvement in average number of daily heartburn events (coefficient − 11.268; 95% CI − 21.356, − 1.180; P = 0.0297).
Safety
In the safety population, a total of 9 AEs were reported; 8 were determined to be treatment emergent [6 (10.9%) subjects], and 1 (1.8%) was not considered treatment emergent. One subject reported an AE of migraine resulting in permanent study medication discontinuation and study withdrawal. The most frequently reported AE was upper respiratory tract infection [3 (5.5%)], reported by 2 subjects (9.5%) with esomeprazole 20 mg/esomeprazole 20 mg and 1 subject (4.5%) with esomeprazole 20 mg/placebo. The next most common AE was gastroenteritis, which was reported by 2 subjects (3.6%), both in the placebo/placebo group.
Discussion
The established association between improvements in reflux symptoms following gastric acid suppression with a PPI has historically been based on statistical comparisons of means from study populations as a whole [7, 11, 13]. Analyzing the relationship between changes in acid suppression and symptom response using individual data demonstrated an increase in percentage of time with intragastric pH > 4 of 17.7%, which provided greater heartburn relief compared with those not achieving this degree of change in pH. A significant positive correlation was observed between both changes in percentage of time with intragastric pH > 4 and median intragastric pH with 24-h heartburn relief (both P < 0.05). The observed significant relationship between the degree of change in time with intragastric pH > 4 and symptom relief suggests that individuals not achieving adequate acid control may benefit from a change in treatment regimen, such as increasing the dose of their acid-suppressive therapy. The relationship between the change in intragastric pH and symptom relief was clinically significant enough to warrant the use of empiric PPI treatment before performing other diagnostic investigations, including endoscopy.
To our knowledge only one previous study investigated this relationship in the context of healing EE [11]. Similar to the current results, Katz et al. reported significant correlations between controlling daytime and nighttime heartburn and acid regurgitation after 28 days of treatment and percentage of time with pH > 4 on day 5 [11]. However, those data were analyzed on a population level rather than individually, which could be important to consider for individuals not fully responding to PPI therapy. While this previous study demonstrated the efficacy of PPIs for reducing acid secretion using population-level data, individual degrees of change in intragastric pH and the relationship to treatment response have not been assessed in a population that is likely to self-treat their symptoms in a way that is consistent with empiric PPI treatment for heartburn.
In the current study, we observed a significant association between complete heartburn resolution and percentage of time with intragastric pH > 4. The previous EE study only found a numerical (nonsignificant) association between these outcomes [11]. Our study also found a significant correlation between epigastric pain and percentage of time with intragastric pH > 4. A significant correlation was also observed between QOLRAD emotional distress, food/drink problems, and physical/social functioning and change in percentage of time with intragastric pH > 4. This is not unexpected, as a previous study observed a reduction in heartburn frequency and improved QOLRAD scores subsequent to potent acid suppression therapy, but that study did not evaluate these findings in relation to intragastric pH [14]. The current study demonstrated that individual changes in percentage of time with esophageal pH < 4 were correlated with improvements in heartburn frequency and severity, QOLRAD food/drink problems, and vitality. This is consistent with the observation that omeprazole effectively reduced both daytime and nighttime esophageal acid exposure in subjects experiencing EE healing [15].
The observed correlation between acid control and heartburn relief occurred in all subjects whether they received active treatment or placebo. These results confirm the wide variability in individual gastric acid secretion as a part of normal gastrointestinal physiology while supporting the observation that a greater decrease in gastric acid secretion is correlated with a greater likelihood of symptomatic improvement. Although there were changes in the amount of time with intragastric pH > 4 for both those who did and did not achieve relief, the difference between these groups was significant, which may represent an area of individual esophageal sensitivity. The substantial variability in pH measurements in the placebo group is a surprising and interesting finding and may help to explain high placebo response rates in these types of studies. This observation might have been missed if these data were not analyzed individually. The mean change from baseline in percentage of time with pH > 4 was 70.2% with esomeprazole 20 mg/esomeprazole 20 mg and 42.8% with esomeprazole 20 mg/placebo compared with 0.78% with placebo/placebo. However, the number of subjects not experiencing heartburn relief despite substantial increases in time with intragastric pH > 4 may suggest that in certain individuals, heartburn can be attributed to factors other than reflux. As a result, identifying those individuals can help determine alternative treatment options beyond acid-suppressive therapy, which is consistent with use of empiric PPI therapy before performing other diagnostic studies, including endoscopic investigation, to diagnose those with heartburn related to acid reflux as well as those whose symptoms are from other causes. The observed dose–response relationship is expected and consistent with previous esomeprazole pharmacodynamic studies [16,17,18,19]. However, this apparent dose–response relationship did not translate proportionally to symptom control; there was a numerically higher rate of heartburn relief with esomeprazole once daily (55.6%) compared with twice daily (43.8%).
The primary limitation of this study was its preliminary nature. As proof-of-concept studies generally have relatively small populations, a more comprehensive study may confirm these results and establish a more sensitive assessment of the relationship between acid control and heartburn relief with different doses and dosing schedules. Future studies in this area may provide important insights into secondary treatment options for incomplete responders to OTC PPIs. In the current study, a larger proportion of subjects in the placebo group were removed from the PP population compared with the active treatment groups since subjects in the placebo group were more likely to require rescue medication, thereby violating the definition of the PP population. However, in analyses conducted with the total population, the direction of the relationship between 24-h heartburn relief and change from baseline in the percentage of time with intragastric pH > 4 was consistent with that of the PP population, despite no longer being statistically significant. We hope that our observations will improve the design of future protocols and that the information gathered from this study will be considered in future research in this area.
Conclusion
This pilot, proof-of-concept study demonstrated that the likelihood of individuals experiencing symptomatic relief improves relative to reductions in gastric acidity. This observation was valid across all subjects, including those receiving placebo. As expected, subjects in the esomeprazole cohorts experienced notable increases in symptomatic improvement, indicating that the endpoints are consistent with the pharmacotherapeutic effect of PPIs. Individuals experiencing a greater degree of change from baseline in percentage of the day with intragastric pH > 4 were more likely to experience reflux symptom relief or resolution than individuals with a lesser degree of change of time with intragastric pH > 4. Assessing individual-subject data helped interpret both the observed therapeutic failures with acid-suppressive therapy and placebo response in symptomatic heartburn. The data we present suggest that assessing changes in 24-h intragastric pH may be useful for deciding whether to increase the PPI dose or to explore alternative causes of heartburn in those not responding to empiric OTC PPI therapy. Evaluating the dose–response relationship with individual response levels would be useful in clinical management and can provide guidance for explaining the differential diagnosis of heartburn in individual patients. These results also support the use of empiric PPI treatment as a management strategy for frequent heartburn in the OTC setting.
References
Savarino V, Di Mario F, Scarpignato C. Proton pump inhibitors in GORD: an overview of their pharmacology, efficacy and safety. Pharmacol Res. 2009;59(3):135–53.
Katz PO, Gerson LB, Vela MF. Guidelines for the diagnosis and management of gastroesophageal reflux disease. Am J Gastroenterol. 2013;108(3):308–28.
Fiorucci S, Santucci L, Chiucchiu S, Morelli A. Gastric acidity and gastroesophageal reflux patterns in patients with esophagitis. Gastroenterology. 1992;103(3):855–61.
Rohss K, Hasselgren G, Hedenstrom H. Effect of esomeprazole 40 mg vs omeprazole 40 mg on 24-hour intragastric pH in patients with symptoms of gastroesophageal reflux disease. Dig Dis Sci. 2002;47(5):954–8.
Franco MT, Salvia G, Terrin G, et al. Lansoprazole in the treatment of gastro-oesophageal reflux disease in childhood. Dig Liver Dis. 2000;32(8):660–6.
Katz PO, Hatlebakk JG, Castell DO. Gastric acidity and acid breakthrough with twice-daily omeprazole or lansoprazole. Aliment Pharmacol Ther. 2000;14(6):709–14.
Bell NJ, Burget D, Howden CW, Wilkinson J, Hunt RH. Appropriate acid suppression for the management of gastro-oesophageal reflux disease. Digestion. 1992;51(Suppl 1):59–67.
Labenz J, Tillenburg B, Peitz U, et al. Efficacy of omeprazole one year after cure of Helicobacter pylori infection in duodenal ulcer patients. Am J Gastroenterol. 1997;92(4):576–81.
Sachs G, Shin JM, Briving C, Wallmark B, Hersey S. The pharmacology of the gastric acid pump: the H+,K+ ATPase. Annu Rev Pharmacol Toxicol. 1995;35:277–305.
Andersson T, Rohss K, Bredberg E, Hassan-Alin M. Pharmacokinetics and pharmacodynamics of esomeprazole, the S-isomer of omeprazole. Aliment Pharmacol Ther. 2001;15(10):1563–9.
Katz PO, Ginsberg GG, Hoyle PE, Sostek MB, Monyak JT, Silberg DG. Relationship between intragastric acid control and healing status in the treatment of moderate to severe erosive oesophagitis. Aliment Pharmacol Ther. 2007;25(5):617–28.
Wiklund I, Junghard O, Grace E, et al. Quality of life in reflux and dyspepsia. Development and psychometric documentation of a disease-specific questionnaire (QOLRAD) (abstract G0199). Gastroenterology. 1998;114(suppl 1):A49.
Johnson DA, Katz PO, Levine D, et al. Prevention of relapse of healed reflux esophagitis is related to the duration of intragastric pH > 4. J Clin Gastroenterol. 2010;44(7):475–8.
Kahrilas PJ, Jonsson A, Denison H, Wernersson B, Hughes N, Howden CW. Impact of regurgitation on health-related quality of life in gastro-oesophageal reflux disease before and after short-term potent acid suppression therapy. Gut. 2014;63(5):720–6.
Dehn TC, Shepherd HA, Colin-Jones D, Kettlewell MG, Carroll NJ. Double blind comparison of omeprazole (40 mg od) versus cimetidine (400 mg qd) in the treatment of symptomatic erosive reflux oesophagitis, assessed endoscopically, histologically and by 24 h pH monitoring. Gut. 1990;31(5):509–13.
Lind T, Rydberg L, Kyleback A, et al. Esomeprazole provides improved acid control vs. omeprazole in patients with symptoms of gastro-oesophageal reflux disease. Aliment Pharmacol Ther. 2000;14(7):861–7.
Rohss K, Wilder-Smith C, Naucler E, Jansson L. Esomeprazole 20 mg provides more effective intragastric acid control than maintenance-dose rabeprazole, lansoprazole or pantoprazole in healthy volunteers. Clin Drug Investig. 2004;24(1):1–7.
Wilder-Smith C, Backlund A, Eckerwall G, Lind T, Fjellman M, Rohss K. Effect of increasing esomeprazole and pantoprazole doses on acid control in patients with symptoms of gastro-oesophageal reflux disease: a randomized, dose-response study. Clin Drug Investig. 2008;28(6):333–43.
Wilder-Smith C, Lind T, Lundin C, Naucler E, Nilsson-Pieschl C, Rohss K. Acid control with esomeprazole and lansoprazole: a comparative dose-response study. Scand J Gastroenterol. 2007;42(2):157–64.
Acknowledgements
The authors wish to acknowledge the clinicians who previously worked on this study, particularly James T. Angello, PharmD, and David Savastano, PhD. Their support was integral to the success of the study. We also thank the participants of the study.
Funding
This study was sponsored by Pfizer Consumer Healthcare, Madison, NJ, USA. The study sponsor also funded the article processing charges and Open Access fee. All authors had full access to all of the data in this study and take complete responsibility for the integrity of the data and accuracy of the data analysis.
Medical Writing and Editorial Assistance
Medical writing support was provided by Dennis Stancavish of Peloton Advantage, LLC, and was funded by Pfizer.
Authorship
All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this article, take responsibility for the integrity of the work as a whole, and have given their approval for this version to be published.
Disclosures
Philip B. Miner, Jr has served as a consultant, advisory board member, and principal investigator for Pfizer. David A. Johnson has served as a consultant to Pfizer and Proctor & Gamble and an advisory board member for Medscape/WebMD. Philip O. Katz is a consultant for Torax Medical. Jing Li is an employee of Pfizer Consumer Healthcare. Sergio C. Gatoulis is an employee of and owns stock in Pfizer. Charles Pollack was an employee of Pfizer Consumer Healthcare at the time of the study. Charles Pollack is now an employee of Avrio Health.
Compliance with Ethics Guidelines
This study complied with the ethical principles of the Declaration of Helsinki and all International Conference for Harmonisation Good Clinical Practice Guidelines. The final protocol, any amendments, and informed consent forms were reviewed and approved on December 9, 2015, by an independent institutional review board at the investigational center (Oklahoma Foundation for Digestive Research; Oklahoma City, OK, USA). Informed consent was obtained from all individual participants included in the study.
Data Availability
Upon request, and subject to certain criteria, conditions and exceptions, see https://www.pfizer.com/science/clinical-trials/trial-data-and-results for more information, Pfizer will provide access to individual de-identified participant data from Pfizer-sponsored global interventional clinical studies conducted for medicines, vaccines, and medical devices (1) for indications that have been approved in the USA and/or EU or (2) in programs that have been terminated (i.e., development for all indications has been discontinued). Pfizer will also consider requests for the protocol, data dictionary, and statistical analysis plan. Data may be requested from Pfizer trials 24 months after study completion. The de-identified participant data will be made available to researchers whose proposals meet the research criteria and other conditions, and for which an exception does not apply, via a secure portal. To gain access, data requestors must enter into a data access agreement with Pfizer.
Open Access
This article is distributed under the terms of the Creative Commons Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/), which permits any noncommercial use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
Author information
Authors and Affiliations
Corresponding author
Additional information
Enhanced digital features
To view enhanced digital features for this article go to https://doi.org/10.6084/m9.figshare.7017266.
Rights and permissions
This article is published under an open access license. Please check the 'Copyright Information' section either on this page or in the PDF for details of this license and what re-use is permitted. If your intended use exceeds what is permitted by the license or if you are unable to locate the licence and re-use information, please contact the Rights and Permissions team.
About this article
Cite this article
Miner, P.B., Johnson, D.A., Katz, P.O. et al. Pilot, Randomized, Blinded, Placebo-Controlled Trial Investigating the Correlation Between Acid Control and Heartburn Relief with 14 Days of Esomeprazole Treatment. Adv Ther 35, 2024–2040 (2018). https://doi.org/10.1007/s12325-018-0792-z
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12325-018-0792-z