Abstract
Introduction
The immune colloidal gold (ICG) method of measuring thyroid-stimulating hormone (TSH) is a rapid and easy-to-perform test, allowing off-site measurements. This study compared the clinical utility of the first ICG-based qualitative and quantitative TSH test methods in China with the third-generation serum TSH assay used worldwide.
Methods
Fingertip and venous blood was collected within 30 min from 283 patients initially suspected of hypothyroidism. TSH was measured in fingertip blood using ICG-based qualitative quantitative tests. Serum TSH in venous blood was tested using the third-generation serum TSH assay. Correlations between systems were tested by kappa or Spearman correlation coefficients.
Results
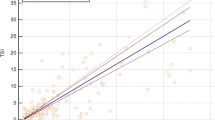
Compared with the third-generation serum TSH assay, the ICG-qualitative TSH test kit had a kappa coefficient of 0.86, a sensitivity of 85.00%, and a specificity of 99.38% in screening for hypothyroidism. The percentages of false negatives and false positives among all subjects were 6.38% and 0.35% respectively; the total consistency rate of the two methods was 93.26%. When compared with the third-generation serum TSH assay, the ICG-quantitative TSH analysis system had a Spearman correlation coefficient of 0.91, a sensitivity of 88.43%, and a specificity of 98.77%. The percentages of false negatives and false positives among all subjects were 4.95% and 0.71%, respectively; the total consistency rate of the two methods was 94.35%.
Conclusion
Both ICG-based assays are easier and faster to perform than the third-generation, laboratory-based serum TSH assay method. The ICG-based methods showed acceptable performance in the simplified screening for hypothyroidism.
Trial registration
ClinicalTrials.gov identifier, NCT01921452.
Funding
Merck Serono Co., Ltd.




Similar content being viewed by others
References
Koulouri O, Moran C, Halsall D, Chatterjee K, Gurnell M. Pitfalls in the measurement and interpretation of thyroid function tests. Best Pract Res Clin Endocrinol Metab. 2013;27:745–62.
Stockigt J. Assessment of thyroid function: towards an integrated laboratory-clinical approach. Clin Biochem Rev. 2003;24(4):109–22.
Baloch Z, Carayon P, Conte-Devolx B, et al. Guidelines Committee, National Academy of Clinical Biochemistry, laboratory medicine practice guidelines. Laboratory support for the diagnosis and monitoring of thyroid disease. Thyroid. 2003;13:3–126.
Kopp P. The thyroid: fundamental and clinical text. In: Braverman LE, Utiger RD, editors. Thyroid hormone synthesis. 9th ed. Philadelphia: Lippincott Williams and Wilkins; 2005. p. 52.
Cooper DS, Doherty GM, Haugen BR, et al. Management guidelines for patients with thyroid nodules and differentiated thyroid cancer. Thyroid. 2006;16:109–42.
Aghini-Lombardi F, Antonangeli L, Martino E, et al. The spectrum of thyroid disorders in anmiodine-deficient community: the Pescopagano survey. J Clin Endocrinol Metab. 1999;84:561–6.
Walsh JP, Bremner AP, Bulsara MK, et al. Subclinical thyroid dysfunction and blood pressure: a community-based study. Clin Endocrinol (Oxf). 2006;65:486–91.
Cooper DS, Biondi B. Subclinical thyroid disease. Lancet. 2012;379(9821):1142–54.
Teng W, Shan Z, Teng X, et al. Effect of iodine intake on thyroid diseases in China. N Engl J Med. 2006;354:2783–93.
Yu-long Cong. Development tendency and challenge of ecsomatics in our country. Continuing Medical Education. 2007;21:1–6.
Bi-qiong Dong. Conception on construction of resource sharing platform of large-scale instrument and equipment in basic laboratories. J Occup Health Damage. 2015;30:64–5.
Shoham Z, Katz E, Blickstein I, et al. New immunochemical method for rapid detection of human choriogonadotropin in urine. Clin Chem. 1987;33:800–2.
Foster SA, Goode JV, Small RE. Home blood glucose monitoring. Ann Pharmacother. 1999;33:355–63.
Wang T, Lu H, Ruan M. Evaluation on clinical utility of immune colloidal gold method for rapid qualitative and quantitative test of thyroid stimulating hormone. In: Abstract presented at the 11th Asia Oceania Thyroid Association Congress (AOTA), Kochi, 25–28 Sep 2014, abstract no. P61.
Bouland DL, Doram K. Thyroid function tests-the next generation. West J Med. 1994;160:248–9.
Brabant G, Prank K, Hoang-Vu C, Hesch RD, von zur Muhlen A. Hypothalamic regulation of pulsatile thyrotopin secretion. J Clin Endocrinol Metab. 1991;72:145–50.
Brabant G, Prank K, Ranft U, et al. Physiological regulation of circadian and pulsatile thyrotropin secretion in normal man and woman. J Clin Endocrinol Metab. 1990;70:403–9.
Garber JR, Cobin RH, Gharib H, et al. Clinical practice guidelines for hypothyroidism in adults: cosponsored by the American Association of Clinical Endocrinologists and the American Thyroid Association. Endocr Pract. 2012;18:988–1028.
von Lode P, Hagren V, Palenius T, et al. One-step quantitative thyrotropin assay for the detection of hypothyroidism in point-of-care conditions. Clin Biochem. 2003;36:121–8.
Hejun Yang. The number of patients with thyroid disease may over 200 million in China. Shanghai Med Pharm J. 2013;12:44.
Faulk WP, Taylor GM. An immunocolloid method for the electron microscope. Immunochemistry. 1971;8:1081–3.
Wenchuan Zhu, Fande Kong, Xiangme Lin, et al. Prospect and application of immune colloidal gold technique. Biotechnol Bull. 2010;4:81–7.
Hale CA, Fleiss JL. Interval estimation under two study designs for kappa with binary classifications. Biometrics. 1993;49:523–34.
Wang T, Shan F, Lu H. Performance assessment of quantitative thyroid stimulating hormone by immune colloidal gold assay. Labeled Immunoassays and Clinical Medicine 2016;23:567–70, 78.
Vassilopoulou-Sellin R, Schultz PN, Haynie TP. Clinical outcome Of patients with papillary thyroid carcinoma who have recurrence after initial radioactive iodine therapy. Cancer. 1996;78:493–501.
Stagnaro-Green A, Abalovich M, Alexander E, et al. American Thyroid Association taskforce on thyroid disease during pregnancy and postpartum, guidelines of the American thyroid association for the diagnosis and management of thyroid disease during pregnancy and postpartum. Thyroid. 2011;21:1081–125.
Acknowledgments
Sponsorship and article processing charges for this study were funded by Merck Serono Co., Ltd., Beijing, China, an affiliate of Merck KGaA, Darmstadt, Germany. Merck Serono Co., Ltd., was also responsible for protocol development, data management and analysis, and manuscript reviewing. The authors would like to thank the physicians from Shanghai 6th People’s Hospital and staff members from Merck Serono Co., Ltd., who contributed greatly to this study. All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this manuscript, take responsibility for the integrity of the work as a whole, and have given final approval for the version to be published.
Disclosures
Tingting Wang has no conflicts of interest or financial ties to disclose. Shiwei Sheng has no conflicts of interest or financial ties to disclose. Meifang Ruan is an employee of Merck Serono Co., Ltd., Beijing, China, an affiliate of Merck KGaA, Darmstadt, Germany. Jing Yan is an employee of Merck Serono Co., Ltd., Beijing, China, an affiliate of Merck KGaA, Darmstadt, Germany. Jianying Gu has no conflicts of interest or financial ties to disclose. Yumin Jiang has no conflicts of interest or financial ties to disclose. Yunchao Gao has no conflicts of interest or financial ties to disclose. Hankui Lu has no conflicts of interest or financial ties to disclose.
Compliance with Ethics Guidelines
This study was registered at ClinicalTrials.gov, under identification no. NCT01921452. The study protocol was approved by the Committees on Ethics and Research Protocols of Shanghai Jiaotong University Affiliated Sixth People’s Hospital. All procedures followed were in accordance with the ethical standards of the responsible committee on human experimentation (institutional and national) and with the Helsinki Declaration of 1964, as revised in 2013. All subjects provided voluntary written informed consent prior to performing trial-related activities.
Author information
Authors and Affiliations
Corresponding author
Additional information
Enhanced Content
To view enhanced content for this article go to http://www.medengine.com/Redeem/1295F0607DFE8C70.
Rights and permissions
About this article
Cite this article
Wang, T., Sheng, S., Ruan, M. et al. Clinical Evaluation of the Immune Colloidal Gold Method for Rapid Qualitative and Quantitative Measurement of Thyroid-Stimulating Hormone as an Assay for Hypothyroidism. Adv Ther 33, 2001–2011 (2016). https://doi.org/10.1007/s12325-016-0401-y
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12325-016-0401-y