Abstract
Introduction
There is scant real-world information on switching treatment for anemia associated with chronic kidney disease (CKD) from methoxy polyethylene glycol-epoetin beta (PEG-Epo) to darbepoetin alfa (DA). TRANSFORM was a multi-center, observational study designed to describe the time course of hemoglobin (Hb) concentration (primary outcome measure) and other parameters of clinical management of anemia in European hemodialysis patients in clinical practice before and after a switch from PEG-Epo to DA.
Methods
Eligible subjects were adult patients with CKD dialyzed at European dialysis centers for ≥26 weeks and treated with PEG-Epo for ≥14 weeks immediately prior to being switched to DA and no earlier than January 2011. Erythropoiesis-stimulating agent doses and Hb values were recorded for the 14-week pre-switch and 26-week post-switch periods.
Results
Of the 1,027 eligible patients enrolled at 42 hemodialysis centers in 7 European countries, 785 were included in analyses. Mean (95% confidence interval [CI]) Hb was generally stable: 11.19 (11.11, 11.26), 11.48 (11.40, 11.57), and 11.29 (11.20, 11.37) g/dL at month −1 pre-switch and months 3 and 6 post-switch, respectively. The geometric mean (95% CI) PEG-Epo dose at month −1 was 27.4 (26.0, 28.8) µg/week; DA dose was 29.4 (27.9, 30.9), 23.3 (21.9, 24.9), and 25.6 (24.1, 27.1) µg/week at months 1, 4, and 6, respectively. The geometric mean (95% CI) dose ratio at switching was 1.06 (1.01, 1.11). When stratifying by dose-ratio categories <0.8, 0.8–1.2, and >1.2 at switching, mean DA dose and Hb converged within narrow ranges by month 6 post-switch: 23.9–27.0 µg/week and 11.1–11.5 g/dL, respectively. Hb excursions <10 g/dL were less frequent post-switch versus pre-switch.
Conclusion
Mean Hb values remained within a narrow range following switching from PEG-Epo to DA in this population of hemodialysis patients. Time trends of mean Hb and DA dose indicate that physicians titrated DA doses post-switch, to attain Hb concentrations comparable to those attained pre-switch with PEG-Epo.
Funding
Amgen (Europe) GmbH, Zug, Switzerland.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Anemia is a complication of chronic kidney disease (CKD), and is twice as common in patients with any stage of CKD as in the general population [1]. Anemia of CKD increases in prevalence and severity as kidney function declines [2], with over 90% of patients who progress to renal replacement therapy becoming anemic [3].
Anemia of CKD results primarily from decreased production of endogenous erythropoietin (EPO) by the kidneys [4]. There is a clear clinical rationale for correcting low hemoglobin (Hb) levels in patients with CKD, as persistent anemia is associated with increased risk of cardiac complications and mortality and impaired quality of life [5–7]. The introduction of exogenous erythropoiesis-stimulating agents (ESAs) to clinical practice transformed the care of patients with CKD by ameliorating anemia, reducing transfusion requirements and improving quality of life [8].
The first-generation recombinant ESAs (epoetin alfa and beta) were short-acting, generally requiring administration three times per week [9]. While short-acting originator (and, more recently, their biosimilar) ESAs remain available, using the subsequently developed longer-acting ESAs, which require less frequent dosing, appears to reduce the burden of administration on patients, caregivers and healthcare professionals [10]. The two longer-acting ESAs, darbepoetin alfa (DA) and methoxy polyethylene glycol-epoetin beta (PEG-Epo), combine a significantly increased half-life with a lower binding affinity for the EPO receptor, allowing them to stimulate erythropoiesis for longer periods and to be administered less frequently [9, 11]. DA, launched in 2001, has five N-linked oligosaccharide chains rather than the three contained in short-acting epoetins, conferring an approximately threefold longer serum half-life and mean residence time, and is approved for administration to hemodialysis patients once a week (QW) or once every 2 weeks (Q2W) [9, 11, 12]. Compared with epoetin beta, PEG-Epo, launched in 2009, has an increased serum half-life that is due to the addition of methoxy polyethylene glycol (PEG), and is approved for administration to patients on dialysis Q2W or once a month (QM) [9, 11, 13].
When switching patients from short-acting ESAs to DA or PEG-Epo, clinicians have reference to the dose conversion schedules recommended in the DA and PEG-Epo labels [12, 13]. The PEG-Epo label also provides recommended dose ranges when converting patients from DA to PEG-Epo [13], and Hb and dose data for patients switching from DA to PEG-Epo are available from randomized clinical studies [14, 15] and from observational studies in routine clinical practice [16]. In contrast, there is no label guidance on switching patients from PEG-Epo to DA, and to our knowledge only a single study, published only in abstract form, has examined outcomes associated with switching from PEG-Epo to DA [17]. The objectives of the Observational Cohort Study of Aranesp® Use in EU Haemodialysis Patients Switched From Mircera® (TRANSFORM) study were primarily to describe the time course of Hb concentration in EU hemodialysis patients in real-world clinical practice switched from PEG-Epo to DA, and secondarily to describe other parameters of clinical management of anemia in this group of patients.
Methods
The TRANSFORM study was designed as a longitudinal cohort analysis of hemodialysis patients switched from PEG-Epo to DA. Data collection for the pre-switch period was entirely retrospective, while data collection for the post-switch period could have been either fully retrospective or partly prospective depending on whether or not the 26-week post-switch period had been completed by the date of site initiation. The study was entirely non-interventional, with no intention to alter the clinical management of the enrolled patients. To ensure that the switch from PEG-Epo was independent of a site’s participation in the TRANSFORM study, each site was informed of the study and approached for enrollment only after it had been confirmed that switching of potential study subjects was complete at that site. This article does not contain any experimental studies with human or animal subjects performed by any of the authors.
Eligible subjects were adult (aged 18 years or over) hemodialysis patients with CKD, who were switched from PEG-Epo to DA during or after January 2011. Patients were required to fulfill the following additional inclusion criteria: receiving hemodialysis at a European dialysis center for at least 26 weeks immediately prior to switching from PEG-Epo to DA; treatment with PEG-Epo for at least 14 weeks immediately prior to being switched to DA; receipt of at least 1 dose of DA post-switch; and provision of written informed consent, according to local requirements. Patients were excluded if they had: been administered more than 2 doses of an ESA other than PEG-Epo in the 7 to 14 weeks immediately prior to switching from PEG-Epo to DA, and/or an ESA other than PEG-Epo during the 6 weeks immediately prior to switching to DA; received chemotherapy during the 14 weeks prior to the switch; undergone major surgery within 14 weeks prior to the switch; or received a red blood cell (RBC) transfusion within 14 weeks prior to the switch.
Each enrolling dialysis center was expected to enroll a minimum of 20 patients into the study. The overall study recruitment target was approximately 1,000 patients, and all subjects were enrolled between August 28, 2012 and July 3, 2013.
Data on clinical and laboratory parameters relating to CKD management were abstracted from patient records and entered by study center staff into an anonymized study-specific central database using an electronic case report form provided by the study sponsor, Amgen (Europe) GmbH. Data were collected from 14 weeks prior to the switch from PEG-Epo until 26 weeks post-switch, regardless of whether subjects remained on DA or converted to an alternative therapy. Data capture ceased at the date of kidney transplant, death or loss to follow-up if these occurred before week 26 post-switch. Data were checked for impossible or implausible values by an automatic review program built into the database software.
The primary outcome measure of the TRANSFORM study was Hb concentration at monthly intervals. Secondary outcome measures included PEG-Epo and DA doses over time, the dose ratio of DA to PEG-Epo at switching, and the incidence of Hb excursions below 10 g/dL and above 12 g/dL. These Hb-concentration thresholds were selected for examination of Hb excursions because 10–12 g/dL is the target Hb range according to the guidance in the product labels for both PEG-Epo and DA [12, 13], and this target range is incorporated in the position statement on anemia management in CKD of the European Renal Best Practice Advisory Board [18]. Results were analyzed for all patients as well as for patient subgroups stratified by DA:PEG-Epo dose ratio at the time of switching in the following three categories: <0.8, 0.8–1.2 and >1.2. Data on administration of intravenous (IV) iron supplements and iron repletion (transferrin saturation [TSAT] and serum ferritin) were also collected.
All procedures followed were in accordance with the ethical standards of the responsible committee on human experimentation (institutional and national) and with the Helsinki Declaration of 1975, as revised in 2000 and 2008. Informed consent was obtained from all patients for being included in the study where applicable according to local requirements.
Statistical Methods
As no formal hypothesis was tested in this study, all analyses were descriptive; no tests of statistical significance were performed. Average Hb values were reported using arithmetic means, and mean weekly ESA doses were reported using geometric means due to the skewed nature of the dosing data. Two-sided 95% confidence intervals (CIs) were calculated for mean Hb and weekly ESA doses.
The dose ratio at switching for each subject was calculated by subtracting the log-transformed weekly dose of PEG-Epo at switch from the log-transformed weekly first dose of DA. The geometric mean dose ratio was derived from the exponential of the arithmetic mean of the log-transformed dose ratio.
Analyses were conducted for all eligible enrolled subjects who received at least one dose of DA, completed 26 weeks post-switch on DA, did not receive another ESA during this period, and had at least one Hb measurement per month recorded for a minimum of any 4 months post-switch. Any Hb values from samples drawn within 14 weeks after a RBC transfusion were excluded from analysis. Results are presented for observed data, with no imputation of missing values. Data from this study were analyzed using SAS Statistical Software v9.2 (SAS Institute Inc., Cary, NC, USA).
Results
A total of 1,027 eligible patients were enrolled at 42 hemodialysis centers in seven European countries; more than half (56%) in France (Table 1). Of the 1,027 patients enrolled, 785 (76.4%) were included for analysis. Reasons for exclusion from the analysis set are presented in Fig. 1. The dates of the first and last ESA switch in the cohort were January 26, 2011 and January 30, 2013, respectively. The baseline (i.e., at time of switch) demographic and clinical characteristics of patients included in the analysis are shown in Table 2. The median patient age was 72 (Q1 60, Q3 79) years; 43.8% of patients were female. Approximately, three-quarters (76.4%) of these CKD patients had hypertension; more than a third (39.1%) had diabetes, a quarter (25.0%) had peripheral vascular disease and almost a quarter (24.1%) had coronary artery disease. A history of renal transplantation was reported for 8.2% of patients. Median duration of hemodialysis was 32.0 (Q1 16.0, Q3 57.0) months.
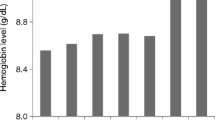
The primary outcome measure, Hb concentration, was generally stable in the post-switch period, rising by less than 0.3 g/dL in the overall cohort from month −1 (i.e., the month pre-switch, when patients were receiving PEG-Epo) to month 3 following the switch to DA, then returning towards pre-switch levels by month 6 post-switch (Fig. 2): mean (95% CI) Hb was 11.19 (11.11, 11.26) g/dL, 11.48 (11.40, 11.57) g/dL, and 11.29 (11.20, 11.37) g/dL at months −1, 3, and 6, respectively.
As shown in Fig. 2, the subgroup analysis stratified by ESA dose ratio at switch revealed that the change in mean monthly Hb concentration in the post-switch period was driven by patients’ dose ratio at switching. Whereas the elevation in mean monthly Hb concentration at month 3 post-switch was greatest in patients with a dose ratio of >1.2, this trend was reversed in patients with a dose ratio at switch of <0.8, with mean monthly Hb declining from the time of switching to month 4 before rising again to month 6 post-switch. Mean monthly Hb concentration for patients with a dose ratio of 0.8–1.2 was roughly parallel to that of the overall cohort. Notably, despite these differences in mean monthly Hb between dose-ratio subgroups, Hb concentration converged by the end of the post-switch period; by month 6, mean Hb concentration was within the range of 11.1–11.5 g/dL across all three dose-ratio subgroups.
Trends in post-switch DA dosing were roughly the opposite of the trends seen for Hb concentration. In the overall cohort, the geometric mean weekly dose of DA was highest at 1 month post-switch and fell to below pre-conversion dosing levels by month 3 (Fig. 3). The geometric mean (95% CI) PEG-Epo dose at month −1 was 27.4 (26.0, 28.8) µg/week, with ESA dose rising to a peak at month 1 with a DA dose of 29.4 (27.9, 30.9) µg/week, then declining to 23.3 (21.9, 24.9) µg/week at month 4 and reaching 25.6 (24.1, 27.1) µg/week at month 6.
In the subgroup analysis stratified by dose ratio at switching, the reduction in geometric mean weekly dose observed from month 1 to month 3 was greatest in patients with a dose ratio of >1.2 (Fig. 3). The trend in geometric mean weekly DA dose for patients with a dose ratio of 0.8–1.2 was similar to that of the overall cohort. In contrast, geometric mean weekly DA doses for patients with a dose ratio <0.8 did not show the same U-shaped trend as for the other dose-ratio categories, but instead increased over the post-switch period. By month 6, geometric mean DA dose was within the range of 23.9–27.0 µg/week across all three dose-ratio subgroups.
Overall, the geometric mean (95% CI) dose ratio at switching was 1.06 (1.01, 1.11), and more than three-quarters of patients (77.8%) had a dose ratio of ≥0.8 (Table 3). By month 6, the geometric mean (95% CI) dose ratio had decreased to 0.88 (0.83, 0.93), and the proportion of patients with a dose ratio of ≥0.8 had dropped to 63.0%. As shown in Fig. 4, there was considerable variability in dose ratio at switch across countries, with geometric mean (95% CI) dose ratio at switch ranging from 0.72 (0.57, 0.90) in the Netherlands to 1.36 (1.17, 1.59) in Spain.
DA:PEG-Epo dose ratio at switch [average weekly dose of the first DA dose divided by the average weekly dose of PEG-Epo at switch (i.e., μg DA per 1 μg PEG-Epo)] by country. Values are geometric means with 95% confidence intervals. Dose ratio for the only patient in Switzerland was 1.0. DA darbepoetin alfa, PEG-Epo methoxy polyethylene glycol-epoetin beta
As reported in Table 4, Hb concentration excursions below the target range (i.e., <10 g/dL) were generally less frequent in the post-switch period compared to the pre-switch period. Hb excursions above the target range (i.e., >12 g/dL) were slightly more frequent during the middle of the post-switch period compared to the pre-switch period, but by month 6 had dropped to a similar incidence as had been observed pre-switch (Table 4).
Use of IV iron supplementation increased steadily from 67% of subjects at month −3 to 81% by month 3, and then declined to 71% by month 6. The most common iron preparation was saccharated iron oxide, administered to over 80% of subjects receiving IV iron supplementation. Mean weekly iron dose ranged from 255 mg at month −3 to 218 mg at month −1, and was 261 mg at month 6. Mean TSAT was 28.9% in the 3 months pre-switch, 26.9% over months 1–3, and 27.6% over months 4–6. Mean serum ferritin was 487.9 μg/L in the 3 months pre-switch, 459.3 μg/L over months 1–3, and 509.6 μg/L over months 4–6.
Discussion
The primary finding of this observational cohort analysis of hemodialysis patients switched from PEG-Epo to DA in real-world clinical practice was that, overall, stable Hb concentrations were maintained over the post-switch period, with Hb values at month 6 for patients receiving DA very similar to those in the month pre-switch when patients were receiving PEG-Epo. Although changes in Hb concentration were observed across the post-switch period, mean monthly Hb values varied over only a very narrow range over time, and remained within the recommended Hb target range of 10–12 g/dL [12, 13] at all study time points.
Analysis of the dose ratio of DA to PEG-Epo at switch indicated a broad range of dose ratios across countries, perhaps reflective of the lack of guidance in the product labels on the appropriate starting dose ratio when switching from PEG-Epo to DA. Despite the observed high variability in dose ratio at switch, both doses and Hb concentration values converged in the post-switch period. As seen for the time course of mean monthly Hb concentration, geometric mean weekly DA doses at month 6 were similar to the geometric mean weekly PEG-Epo doses in the month prior to the switch. Decreasing DA doses in the post-switch period were also evident in the change in geometric mean dose ratio over time, declining from 1.06 at switch to 0.88 at month 6. The return of ESA dose and Hb concentration in the post-switch period towards values similar to those in the month preceding the switch suggests that physicians were able to successfully titrate DA dose after switching, to achieve the desired Hb concentration.
The dose-ratio subgroup analyses provided additional support for the suggestion that changes in the geometric mean weekly dose of DA post-switch likely indicate titration by physicians to maintain a stable Hb concentration. The reduction in DA dose from month 1 to month 3 post-switch was most pronounced in patients switched at a dosing ratio of >1.2 (i.e., a higher DA dose relative to their pre-switch PEG-Epo dose), and this subgroup showed the highest elevation in mean Hb concentration by month 3. Across all three dose-ratio subgroups, mean monthly Hb converged within the range of 11.1–11.5 g/dL by month 6, supporting the idea of dose titration by physicians. This study was non-interventional and was not intended to be prescriptive of dose conversion ratios for clinical practice; nevertheless, the findings of the subgroup analyses may suggest that the conversion ratio should be <1.2, in a population clinically similar to the sample.
As previously noted, there are scant published data on clinical parameters associated with switching from PEG-Epo to DA. The single previously published study of a switch from PEG-Epo to DA, reported by Meier in abstract form, compared Hb levels and ESA doses 12 weeks pre-switch and 12 weeks post-switch [17]. In this pilot evaluation, 21 hemodialysis patients from 3 centers were switched from PEG-Epo QM to DA QW, Q2W, or QM. After the switch, more patients reached an Hb level of at least 11 g/dL than had done during the pre-switch period (71.4% vs. 66.7%, respectively) despite a dose decrease of 22%. On the basis of these data, Meier suggested a conversion ratio in the range of 1 µg PEG-Epo to 0.7–0.9 µg DA. A detailed comparison between the findings of TRANSFORM and Meier’s study is precluded by the necessarily limited information reported in the abstract, as well as the differences in sample size and study period. Nevertheless, both studies indicate that a dose conversion ratio above 1.0 is not required when switching from PEG-Epo to DA.
In the present study, excursions of Hb values below the target range of 10–12 g/dL were more common pre-switch compared to the post-switch period. The onset and duration of Hb excursions were not precisely calculable in this observational study, because Hb concentration was recorded per normal clinical practice rather than at a protocol-prescribed frequency.
Results of TRANSFORM are not directly comparable with those of the recently published Aranesp® Efficiency Relative to Mircera® (AFFIRM) study [16], an observational study of European CKD patients switching from DA to PEG-Epo (i.e., the reverse of the switch in TRANSFORM), because the aims and analyses of the two studies were different. In TRANSFORM, dose ratio at switching was a secondary outcome measure calculated across all patients in the analysis set, independently of achieved Hb level. In contrast, the primary goal of AFFIRM was to calculate the population mean maintenance dose conversion ratio, defined as the dose ratio achieving comparable Hb level between pre- and post-switch periods.
The TRANSFORM study has several strengths in terms of helping to fill the information gap regarding clinical parameters in CKD patients switched from PEG-Epo to DA, most notably the enrollment of a large cohort of hemodialysis patients receiving real-world clinical management for CKD-related anemia in several European countries. However, there are limitations in the ability to generalize the findings of this study to the broader hemodialysis patient population. Firstly, although the study population was heterogeneous, more than half of the sample was drawn from a single country (France), so the sample may not be as representative of other European countries. Secondly, findings may not be representative of patients experiencing clinical worsening because the study enrolled hemodialysis patients who were stable, as defined by having not received chemotherapy or a blood transfusion, or had surgery within the 14 weeks prior to the switch from PEG-Epo to DA. Finally, since data were recorded as part of normal clinical practice, not all data points were guaranteed to be available for abstraction. Despite this limitation, a high degree of completeness was achieved, since this dialysis population attended hemodialysis centers several times per week, and the study assessments, which were relevant to routine treatment, thus were likely to be measured as a component of regular clinical care.
The study was not designed to compare ESA doses or to estimate a maintenance dose conversion ratio—the primary outcome measure focused solely on Hb, regardless of ESA dose. Therefore, the dose data collected cannot be extrapolated to the cost of either therapy and do not permit accurate pharmacoeconomic evaluation. Additionally, estimating the costs associated with DA or PEG-Epo is difficult, given the differences in center, hospital and country. The lack of information on actual acquisition cost by center (which can differ significantly from list prices) would further complicate any cost comparisons.
Conclusion
The TRANSFORM study showed that in this cohort of EU hemodialysis patients who switched from PEG-Epo to DA (and who completed 26 weeks of treatment with DA post-switch) mean achieved Hb was maintained within a narrow range over the post-switch observation period. The correspondence over time between mean Hb and mean DA dose provides evidence that physicians adjusted DA doses to achieve Hb concentrations comparable to those attained before switching.
TRANSFORM also indicated that higher DA doses were not required in these patients switching from PEG-Epo. There was considerable variation in the dose ratio of DA to PEG-Epo at the time of switching, which may have been driven by the lack of label guidance on dose conversion from PEG-Epo to DA, in the absence of which dose conversion likely reflected clinicians’ preferences. However, there was no difference in mean weekly ESA dose at the end of the post-conversion period compared with pre-conversion dose, regardless of patients’ dose ratio at switch.
References
Stauffer ME, Fan T. Prevalence of anemia in chronic kidney disease in the United States. PLoS One. 2014;9:e84943.
Astor BC, Muntner P, Levin A, Eustace JA, Coresh J. Association of kidney function with anemia: the Third National Health and Nutrition Examination Survey (1988–1994). Arch Intern Med. 2002;162:1401–8.
Kazmi WH, Kausz AT, Khan S, et al. Anemia: an early complication of chronic renal insufficiency. Am J Kidney Dis. 2001;38:803–12.
Eschbach JW, Adamson JW. Anemia of end-stage renal disease (ESRD). Kidney Int. 1985;28:1–5.
Hörl WH. Anaemia management and mortality risk in chronic kidney disease. Nat Rev Nephrol. 2013;9:291–301.
Eckardt KU, Kim J, Kronenberg F, et al. Hemoglobin variability does not predict mortality in European hemodialysis patients. J Am Soc Nephrol. 2010;21:1765–75.
Gilbertson DT, Ebben JP, Foley RN, Weinhandl ED, Bradbury BD, Collins AJ. Hemoglobin level variability: associations with mortality. Clin J Am Soc Nephrol. 2008;3:133–8.
Macdougall IC. Optimizing the use of erythropoietic agents—pharmacokinetic and pharmacodynamic considerations. Nephrol Dial Transpl. 2002;17(Suppl 5):66–70.
Hörl WH. Differentiating factors between erythropoiesis-stimulating agents: an update to selection for anaemia of chronic kidney disease. Drugs. 2013;73:117–30.
Burnier M, Douchamps JA, Tanghe A, et al. Less frequent dosing of erythropoiesis stimulating agents in patients undergoing dialysis: a European multicentre cost study. J Med Econ. 2009;12:77–86.
Macdougall IC. New anemia therapies: translating novel strategies from bench to bedside. Am J Kidney Dis. 2012;59:444–51.
Aranesp® (darbepoetin alfa) Summary of Product Characteristics. Amgen Europe B.V., Breda, The Netherlands, 6 November 2013. http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Product_Information/human/000332/WC500026149.pdf. Accessed 10 Sept 2014.
Mircera® (methoxy polyethylene glycol-epoetin beta) Summary of Product Characteristics. Roche Registration Ltd., Welwyn Garden City, UK, 6 February 2014. http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Product_Information/human/000739/WC500033672.pdf. Accessed 10 Sept 2014.
Carrera F, Lok CE, de Francisco A, et al. Maintenance treatment of renal anaemia in haemodialysis patients with methoxy polyethylene glycol-epoetin beta versus darbepoetin alfa administered monthly: a randomized comparative trial. Nephrol Dial Transpl. 2010;25:4009–17.
Canaud B, Mingardi G, Braun J, et al. Intravenous C.E.R.A. maintains stable haemoglobin levels in patients on dialysis previously treated with darbepoetin alfa: results from STRIATA, a randomized phase III study. Nephrol Dial Transpl. 2008;23:3654–61.
Choi P, Farouk M, Manamley N, Addison J. Dose conversion ratio in hemodialysis patients switched from darbepoetin alfa to PEG-epoetin beta: AFFIRM study. Adv Ther. 2013;30:1007–17.
Meier P. Switch of ESA therapy from CERA to darbepoetin-alpha in chronic hemodialysis patients: a multicenter experience (Abstract TH-PO441: presented at the American Society of Nephrology Renal Week, Denver, CO, USA, 16–21 November 2010). J Am Soc Nephrol. 2010;21:211A.
Locatelli F, Bárány P, Covic A, et al. Kidney disease: improving global outcomes guidelines on anaemia management in chronic kidney disease: a European Renal Best Practice position statement. Nephrol Dial Transpl. 2013;28:1346–59.
Acknowledgments
Sponsorship and article processing charges for this study were funded by Amgen (Europe) GmbH, Zug, Switzerland. All authors had full access to all of the data in this study and take complete responsibility for the integrity of the data and accuracy of the data analysis Publication management support was provided by Jennifer Corrigan, Medical Writing etc., Oxford, UK. Editorial assistance in the preparation of this manuscript was provided by W. Mark Roberts, Montreal, Canada. Support for publication management and editorial assistance was funded by Amgen (Europe) GmbH. Jan Donck is the guarantor for this article, and takes responsibility for the integrity of the work as a whole. All named authors meet the ICMJE criteria for authorship for this manuscript, take responsibility for the integrity of the work as a whole, and have given final approval for the version to be published.
Contributors
Jan Donck, Jacques Chanliau, Heike Martin, Kyriaki Stamatelou, Nick Manamley, Mourad Farouk, and Janet Addison participated in drafting of the manuscript. Jan Donck, Nick Manamley, Mourad Farouk, and Janet Addison contributed to data interpretation. Kyriaki Stamatelou, Mourad Farouk, and Janet Addison participated in study coordination. Lourdes Gonzalez-Tabares contributed to data management, and Nick Manamley performed the statistical analysis. Janet Addison contributed to study conception and design. All authors approved the final manuscript for publication.
Compliance with ethics guidelines
All procedures followed were in accordance with the ethical standards of the responsible committee on human experimentation (institutional and national) and with the Helsinki Declaration of 1975, as revised in 2000 and 2008. Informed consent was obtained from all patients for being included in the study where applicable according to local requirements. This article does not contain any studies with human or animal subjects performed by any of the authors.
Conflict of interest
Jan Donck has received honoraria from Amgen Inc., Thousand Oaks, CA, USA and Roche Europe, Basel, Switzerland. Lourdes Gonzalez-Tabares and Jacques Chanliau declare no conflict of interest. Heike Martin has received honoraria from Amgen Germany, Munich, Germany, Baxter Germany GmbH, Unterschleissheim, Germany, Shire Germany, Berlin, Germany, and Fresenius Medical Care GmbH, Bad Homburg, Germany. Kyriaki Stamatelou has received honoraria from Amgen Hellas, Maroussi, Greece, Genesis Pharma, Athens, Greece, and Leo SARATH, Copenhagen, Denmark. Nick Manamley, Mourad Farouk and Janet Addison are employees of Amgen (Europe) GmbH, Zug, Switzerland, with Amgen stock ownership.
Open Access
This article is distributed under the terms of the Creative Commons Attribution Noncommercial License which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and the source are credited.
Author information
Authors and Affiliations
Corresponding author
Additional information
Trial registration: ClinicalTrials.gov Identifier: NCT01997892.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (https://creativecommons.org/licenses/by/4.0), which permits use, duplication, adaptation, distribution, and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Donck, J., Gonzalez-Tabares, L., Chanliau, J. et al. Preservation of Anemia Control and Weekly ESA Dosage After Conversion from PEG-Epoetin Beta to Darbepoetin Alfa in Adult Hemodialysis Patients: The TRANSFORM Study. Adv Ther 31, 1155–1168 (2014). https://doi.org/10.1007/s12325-014-0161-5
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12325-014-0161-5