Abstract
Introduction
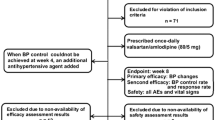
Current hypertension guidelines recommend single-pill combinations because they not only improve convenience and compliance to therapy and thus blood pressure (BP) control, but also reduce health-care costs. This study compared the efficacy and safety of valsartan/amlodipine single-pill combination with nifedipine gastrointestinal therapeutic system (GITS) in Chinese patients with hypertension who were inadequately controlled with monotherapy.
Methods
In this multicenter, open-label, active-controlled, parallel-group study, 564 patients with hypertension not adequately controlled by prior monotherapy were randomized to receive valsartan/amlodipine 80/5 mg or nifedipine GITS 30 mg once daily for 12 weeks.
Results
In the intention-to-treat analysis (n = 540), valsartan/amlodipine (n = 272) showed a least-square mean reduction of −16.6 versus −10.8 mmHg by nifedipine GITS (n = 268; mean between-treatment difference: −5.8 mmHg; P < 0.0001) from baseline to week 12. The corresponding results for mean sitting diastolic BP were −8.6 and −4.6 mmHg, respectively (difference: −4.0 mmHg; P < 0.0001). The percentage of patients achieving the BP target (<140/90 or <130/80 mmHg in the absence or presence of diabetes mellitus, respectively) was significantly higher with valsartan/amlodipine (79.0%) versus nifedipine GITS (57.4%; P < 0.0001). The overall incidence rate of adverse events was lower with valsartan/amlodipine (19.2%) than with nifedipine GITS (29.4%; P = 0.004).
Conclusion
The valsartan/amlodipine 80/5 mg single-pill combination is well tolerated and more effective than nifedipine GITS 30 mg for BP control in Chinese patients with hypertension.





Similar content being viewed by others
References
Kearney PM, Whelton M, Reynolds K, Muntner P, Whelton PK, He J. Global burden of hypertension: analysis of worldwide data. Lancet. 2005;365:217–23.
Liu LS. 2010 Chinese guidelines for the management of hypertension. Zhonghua Xin Xue Guan Bing Za Zhi. 2011;39:579–615.
Hu DY, Liu LS, Yu JM, Yao CH. National survey of blood pressure control rate in Chinese hypertensive outpatients-China STATUS. Zhonghua Xin Xue Guan Bing Za Zhi. 2010;38:230–8.
Julius S, Kjeldsen SE, Weber M, Brunner HR, Ekman S, Hansson L, et al. Outcomes in hypertensive patients at high cardiovascular risk treated with regimens based on valsartan or amlodipine: the VALUE randomised trial. Lancet. 2004;363:2022–31.
Hansson L, Zanchetti A, Carruthers SG, Dahlof B, Elmfeldt D, Julius S, et al. Effects of intensive blood-pressure lowering and low-dose aspirin in patients with hypertension: principal results of the Hypertension Optimal Treatment (HOT) randomised trial. HOT Study Group. Lancet. 1998;351:1755–62.
Leenen FH, Nwachuku CE, Black HR, Cushman WC, Davis BR, Simpson LM, et al. Clinical events in high-risk hypertensive patients randomly assigned to calcium channel blocker versus angiotensin-converting enzyme inhibitor in the antihypertensive and lipid-lowering treatment to prevent heart attack trial. Hypertension. 2006;48:374–84.
Dickson M, Plauschinat CA. Compliance with antihypertensive therapy in the elderly: a comparison of fixed-dose combination amlodipine/benazepril versus component-based free-combination therapy. Am J Cardiovasc Drugs. 2008;8:45–50.
Bangalore S, Kamalakkannan G, Parkar S, Messerli FH. Fixed-dose combinations improve medication compliance: a meta-analysis. Am J Med. 2007;120:713–9.
Tocci G, Volpe M. Modern clinical management of arterial hypertension: fixed or free combination therapies? High Blood Press Cardiovasc Prev. 2011;18(Suppl 1):3–11.
Baser O, Andrews LM, Wang L, Xie L. Comparison of real-world adherence, healthcare resource utilization and costs for newly initiated valsartan/amlodipine single-pill combination versus angiotensin receptor blocker/calcium channel blocker free-combination therapy. J Med Econ. 2011;14:576–83.
Mansia G, De BG, Dominiczak A, Cifkova R, Fagard R, Germano G, et al. 2007 ESH-ESC Guidelines for the management of arterial hypertension: the task force for the management of arterial hypertension of the European Society of Hypertension (ESH) and of the European Society of Cardiology (ESC). Blood Press. 2007;16:135–232.
Chobanian AV, Bakris GL, Black HR, Cushman WC, Green LA, Izzo JL Jr, et al. Seventh report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure. Hypertension. 2003;42:1206–52.
Jamerson K, Weber MA, Bakris GL, Dahlof B, Pitt B, Shi V, et al. Benazepril plus amlodipine or hydrochlorothiazide for hypertension in high-risk patients. N Engl J Med. 2008;359:2417–28.
Dahlof B, Sever PS, Poulter NR, Wedel H, Beevers DG, Caulfield M, et al. Prevention of cardiovascular events with an antihypertensive regimen of amlodipine adding perindopril as required versus atenolol adding bendroflumethiazide as required, in the Anglo-Scandinavian Cardiac Outcomes Trial-Blood Pressure Lowering Arm (ASCOT-BPLA): a multicentre randomised controlled trial. Lancet. 2005;366:895–906.
Jamerson K, Bakris GL, Dahlof B, Pitt B, Velazquez E, Gupte J, et al. Exceptional early blood pressure control rates: the ACCOMPLISH trial. Blood Press. 2007;16:80–6.
Plosker GL, Robinson DM. Amlodipine/valsartan: fixed-dose combination in hypertension. Drugs. 2008;68:373–81.
Ke Y, Zhu D, Hong H, Zhu J, Wang R, Cardenas P, Zhang Y. Efficacy and safety of a single-pill combination of amlodipine/valsartan in Asian hypertensive patients inadequately controlled with amlodipine monotherapy. Curr Med Res Opin. 2010;26:1705–13.
Huang J, Sun NL, Hao YM, Zhu JR, Tu Y, Curt V, Zhang Y. Efficacy and tolerability of a single-pill combination of amlodipine/valsartan in Asian hypertensive patients not adequately controlled with valsartan monotherapy. Clin Exp Hypertens. 2011;33:179–86.
Schrader J, Salvetti A, Calvo C, Akpinar E, Keeling L, Weisskopf M, et al. The combination of amlodipine/valsartan 5/160 mg produces less peripheral oedema than amlodipine 10 mg in hypertensive patients not adequately controlled with amlodipine 5 mg. Int J Clin Pract. 2009;63:217–25.
Acknowledgments
Novartis Pharma AG (Basel, Switzerland) sponsored and funded the study and the present manuscript (writing assistance and article processing charges). The authors acknowledge all investigators and study coordinators at the participating centers and all patients for their commitment to the study. All authors had access to the study data, and reviewed and revised the initial draft and subsequent versions of the manuscript. They had final responsibility for the decision to submit for publication and approved the submitted version. The authors thank Monika Kundu (Novartis) and Adam Tearle (Novartis), professional medical writers, for preparing the draft manuscript and collating and incorporating comments from all authors.
Conflict of interest
Yu-Song He is an employee of Novartis Pharmaceuticals, Beijing, China. Ji-Guang Wang reports receiving grants and lecture and consulting fees from Novartis, the sponsor of the EXAM trial. Wei-Fang Zeng, Liang-Long Chen, Meng Wei, Zhao-Ping Li, Bao-Wei Zhang, and Yan Li declare no conflicts of interest.
Compliance with ethics guidelines
This study was conducted in compliance with the International Conference on Harmonization Guidelines for Good Clinical Practice local regulations and the ethical principles of the Declaration of Helsinki. The study protocol was approved by an independent ethics committee or an institutional review board of each study center. All patients provided written informed consent before undergoing any study procedure.
Author information
Authors and Affiliations
Consortia
Corresponding author
Additional information
ClinicalTrials.gov #NCT01167153.
Appendix
Appendix
Participating hospitals with the name of the principal investigator and the number of patients included in the intention-to-treat analysis: Yong Huo, Peking University First Hospital (n = 31); Zhao-Ping Li, Peking University Third Hospital (n = 41); Yundai Chen, Chinese People’s Liberation Army General Hospital (n = 4); Ming Yang, Beijing Fuxing Hospital, Capital Medical University (n = 23); Yuqing Zhang, Beijing Fuwai Hospital (n = 19); Xinchun Yang, Beijing Chaoyang Hospital, Capital Medical University (n = 24); Ji-Guang Wang, Shanghai Ruijin Hospital (n = 38); Yong Li, Shanghai Huashan Hospital (n = 8); Meng Wei, Shanghai Sixth People’s Hospital (n = 39); Farong Shen, Zhejiang Hospital (n = 42); Ningfu Wang, Hangzhou First People’s Hospital (n = 51); Jiyan Chen, Guangdong Province People’s Hospital (n = 23); Yi Luo, Guangzhou First People’s Hospital (n = 22); Shuxian Zhou, Zhongshan University Second Hospital (n = 21); Wei Wu, Guangzhou University of Chinese Medicine First Hospital (n = 35); Liang-Long Chen, Fujian Medical University Affiliated Union Hospital (n = 51); Yuhua Liao, Wuhan Union Hospital (n = 27); Chuanyu Gao, Henan Province People’s Hospital (n = 39).
Rights and permissions
About this article
Cite this article
Wang, JG., Zeng, WF., He, YS. et al. Valsartan/Amlodipine Compared to Nifedipine GITS in Patients with Hypertension Inadequately Controlled by Monotherapy. Adv Ther 30, 771–783 (2013). https://doi.org/10.1007/s12325-013-0048-x
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12325-013-0048-x