Abstract
Introduction
Streptococcus pneumoniae can cause invasive pneumococcal diseases (IPD), such as bacteremic pneumonia, bacteremia, meningitis, and sepsis, and non-IPDs, such as otitis media, nonbacteremic pneumonia, and upper respiratory tract infections. It was estimated in 2000 that, worldwide, S. pneumoniae was responsible for 826,000 deaths annually in children aged between 1 month and 5 years. A 7-valent pneumococcal conjugate vaccine (PCV7) was licensed in 2000 in the USA and in 2001 in Europe.
Methods
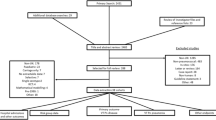
A literature search was performed in PubMed to identify studies assessing the impact of routine childhood PCV7 vaccination on pneumococcal morbidity and mortality. Here, the impact on IPD is reported.
Results
A total of 37 articles reporting impact data on IPD were included in this review: four from Australia, 17 from western Europe, and 16 from North America. In vaccine-eligible children in the postvaccination period, a reduction ranging from 39.9% in Spain to 99.1% in the USA in vaccine-type (VT) IPD incidence, compared with the prevaccination period, was reported in 18 studies. All but one of the 30 studies assessing the impact of PCV7 on all-type IPD reported a reduction ranging from 1.7% in Spain to 76.3% in Australia. In addition, the majority of studies reported reductions in VT and all-type IPD incidence in age groups that were not vaccine eligible.
Conclusions
The results from this review illustrate that PCV7 has had a significant impact on IPD across all ages through its use in pediatric immunization programs. With the introduction of 13-valent pneumococcal conjugate vaccine (PCV13) further reductions in the incidence of IPD due to the six additional serotypes included, as well as continued protection against IPD due to PCV7 serotypes may be expected. Robust surveillance systems are essential for the evaluation of the impact of PCV13 on all-type IPD and for monitoring the evolution of non-VT IPD.
Article PDF
Similar content being viewed by others
Avoid common mistakes on your manuscript.
References
Johnson HL, Deloria-Knoll M, Levine OS, et al. Systematic evaluation of serotypes causing invasive pneumococcal disease among children under five: the pneumococcal global serotype project. PLoS Med. 2010;7:e1000348.
Frenck RW Jr, Yeh S. The development of 13-valent pneumococcal conjugate vaccine and its possible use in adults. Expert Opin Biol Ther. 2012;12:63–77.
O’Brien KL, Wolfson LJ, Watt JP, et al. Burden of disease caused by Streptococcus pneumoniae in children younger than 5 years: global estimates. Lancet. 2009;374:893–902.
Pebody RG, Hellenbrand W, D’Ancona F, Ruutu P. Pneumococcal disease surveillance in Europe. Euro Surveill. 2006;11:171–8.
Rosen JB, Thomas AR, Lexau CA, et al. CDC Emerging Infectious Program Network. Geographic variation in invasive pneumococcal disease following pneumococcal conjugate vaccine introduction in the United States. Clin Infect Dis. 2011;53:137–143.
Fitzwater SP, Chandran A, Santosham M, Johnson HL. The worldwide impact of the seven-valent pneumococcal conjugate vaccine. Pediatr Infect Dis J. 2012;31:501–508.
Rose M, Zielen S. Impact of infant immunization programs with pneumococcal conjugate vaccine in Europe. Expert Rev Vaccines. 2009;8:1351–1364.
Zangeneh TT, Baracco G, Al-Tawfiq JA. Impact of conjugate pneumococcal vaccines on the changing epidemiology of pneumococcal infections. Expert Rev Vaccines. 2011;10:345–353.
World Health Organization. Pneumococcal conjugate vaccine for childhood immunization — WHO position paper. Wkly Epidemiol Rec. 2007;82:93–104.
Rendi-Wagner P, Paulke-Korinek M, Kundi M, et al. National paediatric immunization program of high risk groups: no effect on the incidence of invasive pneumococcal diseases. Vaccine. 2009;27:3963–3968.
Hanquet G, Lernout T, Vergison A, et al. Belgian IPD Scientific Committee. Impact of conjugate 7-valent vaccination in Belgium: addressing methodological challenges. Vaccine. 2011;29:2856–2864.
Harboe ZB, Valentiner-Branth P, Benfield TL, et al. Early effectiveness of heptavalent conjugate pneumococcal vaccination on invasive pneumococcal disease after the introduction in the Danish Childhood Immunization Programme. Vaccine. 2010;28:2642–2647.
Rückinger S, van der Linden M, Reinert RR, von Kries R, Burckhardt F, Siedler A. Reduction in the incidence of invasive pneumococcal disease after general vaccination with 7-valent pneumococcal conjugate vaccine in Germany. Vaccine. 2009;27:4136–4141.
Rodenburg GD, de Greeff SC, Jansen AG, et al. Effects of pneumococcal conjugate vaccine 2 years after its introduction, the Netherlands. Emerg Infect Dis. 2010;16:816–823.
Vestrheim DF, Lovoll O, Aaberge IS, et al. Effectiveness of a 2 + 1 dose schedule pneumococcal conjugate vaccination programme on invasive pneumococcal disease among children in Norway. Vaccine. 2008;26:3277–3281.
Vestrheim DF, Hoiby EA, Bergsaker MR, Ronning K, Aaberge IS, Caugant DA. Indirect effect of conjugate pneumococcal vaccination in a 2 + 1 dose schedule. Vaccine. 2010;28:2214–2221.
Ardanuy C, Tubau F, Pallares R, et al. Epidemiology of invasive pneumococcal disease among adult patients in barcelona before and after pediatric 7-valent pneumococcal conjugate vaccine introduction, 1997–2007. Clin Infect Dis. 2009;48:57–64.
Aristegui J, Bernaola E, Pocheville I, et al. Reduction in pediatric invasive pneumococcal disease in the Basque Country and Navarre, Spain, after introduction of the heptavalent pneumococcal conjugate vaccine. Eur J Clin Microbiol Infect Dis. 2007;26:303–310.
Barricarte A, Gil-Setas A, Torroba L, et al. Invasive pneumococcal disease in children younger than 5 years in Navarra, Spain (2000–2005). Impact of the conjugate vaccine [in Spanish]. Med Clin (Barc). 2007;129:41–45.
Calbo E, Diaz A, Canadell E, et al. Invasive pneumococcal disease among children in a health district of Barcelona: early impact of pneumococcal conjugate vaccine. Clin Microbiol Infect. 2006;12:867–872.
Fenoll A, Granizo JJ, Aguilar L, et al. Temporal trends of invasive Streptococcus pneumoniae serotypes and antimicrobial resistance patterns in Spain from 1979 to 2007. J Clin Microbiol. 2009;47:1012–1020.
Guevara M, Barricarte A, Gil-Setas A, et al. Changing epidemiology of invasive pneumococcal disease following increased coverage with the heptavalent conjugate vaccine in Navarre, Spain. Clin Microbiol Infect. 2009;15:1013–1019.
Munoz-Almagro C, Jordan I, Gene A, Latorre C, Garcia-Garcia JJ, Pallares R. Emergence of invasive pneumococcal disease caused by nonvaccine serotypes in the era of 7-valent conjugate vaccine. Clin Infect Dis. 2008;46:174–182.
Perez-Trallero E, Marimon JM, Ercibengoa M, Vicente D, Perez-Yarza EG. Invasive Streptococcus pneumoniae infections in children and older adults in the north of Spain before and after the introduction of the heptavalent pneumococcal conjugate vaccine. Eur J Clin Microbiol Infect Dis. 2009;28:731–738.
Foster D, Walker AS, Paul J, et al. Oxford Invasive Penumococcal Surveillance Group. Reduction in invasive pneumococcal disease following implementation of the conjugate vaccine in the Oxfordshire region, England. J Med Microbiol. 2011;60:91–97.
Miller E, Andrews NJ, Waight PA, Slack MP, George RC. Herd immunity and serotype replacement 4 years after seven-valent pneumococcal conjugate vaccination in England and Wales: an observational cohort study. Lancet Infect Dis. 2011;11:760–768.
Albrich WC, Baughman W, Schmotzer B, Farley MM. Changing characteristics of invasive pneumococcal disease in Metropolitan Atlanta, Georgia, after introduction of a 7-valent pneumococcal conjugate vaccine. Clin Infect Dis. 2007;44:1569–1576.
Black S, France EK, Isaacman D, et al. Surveillance for invasive pneumococcal disease during 2000–2005 in a population of children who received 7-valent pneumococcal conjugate vaccine. Pediatr Infect Dis J. 2007;26:771–777.
Whitney CG, Farley MM, Hadler J, et al. Active Bacterial Core Surveillance of the Emerging Infectious Program Network. Decline in invasive pneumococcal disease after the introduction of protein-polysaccharide conjugate vaccine. N Engl J Med. 2003;348:1737–1746.
Lexau CA, Lynfield R, Danila R, et al. Active Bacterial Core Surveillance Team. Changing epidemiology of invasive pneumococcal disease among older adults in the era of pediatric pneumococcal conjugate vaccine. JAMA. 2005;294:2043–2051.
Hicks LA, Harrison LH, Flannery B, et al. Incidence of pneumococcal disease due to non-pneumococcal conjugate vaccine (PCV7) serotypes in the United States during the era of widespread PCV7 vaccination, 1998–2004. J Infect Dis. 2007;196:1346–1354.
Centers for Disease Control and Prevention. Invasive pneumococcal disease in children 5 years after conjugate vaccine introduction — eight states, 1998–2005. MMWR Morb Mortal Wkly Rep. 2008;57:144–148.
Pilishvili T, Lexau C, Farley MM, et al. Active Bacterial Core Surveillance/Emerging Infectious Program. Sustained reductions in invasive pneumococcal disease in the era of conjugate vaccine. J Infect Dis. 2010;201:32–41.
Hennessy TW, Singleton RJ, Bulkow LR, et al. Impact of heptavalent pneumococcal conjugate vaccine on invasive disease, antimicrobial resistance and colonization in Alaska Natives: progress towards elimination of a health disparity. Vaccine. 2005;23:5464–5473.
Singleton RJ, Hennessy TW, Bulkow LR, et al. Invasive pneumococcal disease caused by nonvaccine serotypes among alaska native children with high levels of 7-valent pneumococcal conjugate vaccine coverage. JAMA. 2007;297:1784–1792.
Hsu K, Pelton S, Karumuri S, Heisey-Grove D, Klein J. Massachusetts Department of Public Health Epidemiologists. Population-based surveillance for childhood invasive pneumococcal disease in the era of conjugate vaccine. Pediatr Infect Dis J. 2005;24:17–23.
McBean AM, Park YT, Caldwell D, Yu X. Declining invasive pneumococcal disease in the US elderly. Vaccine. 2005;23:5641–5645.
Pulido M, Sorvillo F. Declining invasive pneumococcal disease mortality in the United States, 1990–2005. Vaccine. 2010;28:889–892.
Simonsen L, Taylor RJ, Young-Xu Y, Haber M, May L, Klugman KP. Impact of pneumococcal conjugate vaccination of infants on pneumonia and influenza hospitalization and mortality in all age groups in the United States. mBio. 2011;2:e00309–00310.
Tsigrelis C, Tleyjeh IM, Lahr BD, Nyre LM, Virk A, Baddour LM. Decreases in case-fatality and mortality rates for invasive pneumococcal disease in Olmsted County, Minnesota, during 1995–2007: a population-based study. Clin Infect Dis. 2008;47:1367–1371.
Tyrrell GJ, Lovgren M, Chui N, et al. Serotypes and antimicrobial susceptibilities of invasive Streptococcus pneumoniae pre- and post-seven valent pneumococcal conjugate vaccine introduction in Alberta, Canada, 2000–2006. Vaccine. 2009;27:3553–3560.
Kellner JD, Vanderkooi OG, MacDonald J, Church DL, Tyrrell GJ, Scheifele DW. Changing epidemiology of invasive pneumococcal disease in Canada, 1998–2007: update from the Calgary-area Streptococcus pneumoniae Research (CASPER) study. Clin Infect Dis. 2009;49:205–212.
Hanna JN, Humphreys JL, Murphy DM, Smith HV. Invasive pneumococcal disease in non-indigenous people in north Queensland, 2001–2009. Med J Aust. 2010;193:392–396.
Lehmann D, Willis J, Moore HC, et al. The changing epidemiology of invasive pneumococcal disease in aboriginal and non-aboriginal western Australians from 1997 through 2007 and emergence of nonvaccine serotypes. Clin Infect Dis. 2010;50:1477–1486.
Roche PW, Krause V, Cook H, et al. Pneumococcal Working Party of the Communicable Disease Network Australia. Invasive pneumococcal disease in Australia, 2006. Commun Dis Intell. 2008;32:18–30.
Williams SR, Mernagh PJ, Lee MH, Tan JT. Changing epidemiology of invasive pneumococcal disease in Australian children after introduction of a 7-valent pneumococcal conjugate vaccine. Med J Aust. 2011;194:116–120.
Centers for Disease Control and Prevention. National, state, and urban area vaccination coverage among children aged 19–35 months — United States, 2004. MMWR Morb Mortal Wkly Rep. 2005;54:717–721.
Broder KR, MacNeil A, Malone S, et al. Who’s calling the shots? Pediatricians’ adherence to the 2001–2003 pneumococcal conjugate vaccine-shortage recommendations. Pediatrics. 2005;115:1479–1487.
HM Government. data.gov.uk. NHS Information Centre for Health and Social Care. NHS Immunisation Statistics England 2009-10. London: 2010. Available at: http://data.gov.uk/dataset/nhs-immunisation-statistics-england-2009-10. Accessed Jan 21 2013.
Lepoutre A, Varon E, Georges S, Gutmann L, Levy-Bruhl D. Impact of pneumococcal conjugate vaccine on the epidemiology of invasive pneumococcal diseases in France - 1998-2010 [in French]. 2010 [cited Jun 14 2012]. Available at: http://www.invs.sante.fr/surveillance/epibac/donnees_2010/pneumocoque_impact_2010.pdf. Accessed Jun 14 2012.
Office federal de la sante publique et Commission federale pour les vaccinations. Pneumococcal vaccination recommendations for children under five years old. Replacement of 7-valent conjugate vaccine by 13-valent conjugate vaccine [in French]. 2010 [cited Nov 9 2012]; Available from: http://www.bag.admin.ch/themen/medizin/00682/00684/01097/index.html?lang=fr#sprungmarke1_2. Accessed Nov 9 2012.
Esposito S, Tansey S, Thompson A, et al. Safety and immunogenicity of a 13-valent pneumococcal conjugate vaccine compared to those of a 7-valent pneumococcal conjugate vaccine given as a three-dose series with routine vaccines in healthy infants and toddlers. Clin Vaccine Immunol. 2010;17:1017–1026.
Miller E, Andrews NJ, Waight PA, Slack MP, George RC. Effectiveness of the new serotypes in the 13-valent pneumococcal conjugate vaccine. Vaccine. 2011;29:9127–9131.
Author information
Authors and Affiliations
Corresponding author
Additional information
This article is published with open access at Springerlink.com
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 2.0 International License (https://creativecommons.org/licenses/by/2.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
About this article
Cite this article
Myint, T.T.H., Madhava, H., Balmer, P. et al. The Impact of 7-valent Pneumococcal Conjugate Vaccine on Invasive Pneumococcal Disease: A Literature Review. Adv Therapy 30, 127–151 (2013). https://doi.org/10.1007/s12325-013-0007-6
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12325-013-0007-6