Abstract
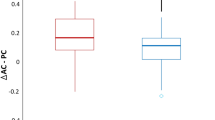
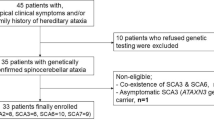
Because of the crucial importance of finding a useful biomarker for further clinical trials in Machado-Joseph disease (MJD), and based on our previous studies, we aimed to evaluate whether the horizontal vestibulo-ocular reflex (VOR) gain could be a reliable neurophysiological biomarker for the clinical onset, severity, and progression of the disease. Thirty-five MJD patients, 11 pre-symptomatic genetically confirmed MJD subjects, and 20 healthy controls underwent a detailed epidemiological and clinical neurological examination including the Scale for the Assessment and Rating of Ataxia (SARA). Their VOR gain was measured using the video Head Impulse Test system. Twenty of the MJD patients were re-tested after a period of 1–3 years. Horizontal VOR gain was abnormal in 92% of MJD, 54% pre-symptomatic, and 0% healthy controls. Horizontal VOR gain in the MJD group was significantly negatively correlated with SARA score in the first (r=0.66, p<0.001) and second (r=0.61, p<0.001) examinations. There was also a significant negative correlation between the percentage of change in horizontal VOR gain and the percentage of change in SARA score across both examinations (r=−0.54, p < 0.05). A regression model of the SARA score with the horizontal VOR gain and disease duration as predictors demonstrated that both the horizontal VOR gain and the disease duration had an independent contribution to the prediction of the SARA score. The horizontal VOR gain seems to be a reliable biomarker for the clinical onset, severity, and progression of MJD and could be used in further clinical studies.





Similar content being viewed by others
Availability of Data and Materials
The data that support the findings of this study are available on request from the corresponding author. The data are not publicly available due to privacy or ethical restrictions.
References
Bettencourt C, Lima M. Machado-Joseph disease: from first descriptions to new perspectives. Orphanet J Rare Dis. 2011;6(1):35. https://doi.org/10.1186/1750-1172-6-35.
do Carmo Costa M. Recent therapeutic prospects for Machado-Joseph disease. Curr Opin Neurol. 2020;33(4):519–26. https://doi.org/10.1097/WCO.0000000000000832.
Oliveira JBL, Martinez ARM, França MC Jr. Pharmacotherapy for the management of the symptoms of Machado-Joseph disease. Expert Opin Pharmacother. 2022;23(15):1687–94. https://doi.org/10.1080/14656566.2022.2135432.
Furtado GV, Oliveira CM, Bolzan G, Saute JA, Saraiva-Pereira ML, Jardim LB. State biomarkers for Machado Joseph disease: validation, feasibility and responsiveness to change. Genet Mol Biol. 2019;42(1):238–51. https://doi.org/10.1590/1678-4685-gmb-2018-0103.
Gordon CR, Joffe V, Vainstein G, Gadoth N. Vestibulo-ocular arreflexia in families with spinocerebellar ataxia type 3 (Machado-Joseph disease). J Neurol Neurosurg Psychiatry. 2003;74(10):1403–6. https://doi.org/10.1136/jnnp.74.10.1403.
Gordon CR, Caspi A, Levite R, Zivotofsky AZ. Mechanisms of vestibulo-ocular reflex (VOR) cancellation in spinocerebellar ataxia type 3 (SCA-3) and episodic ataxia type 2 (EA-2). In: Progress in Brain Research, vol. 171. Elsevier; 2008. p. 519–25. https://doi.org/10.1016/S0079-6123(08)00674-2.
Gordon CR, Zivotofsky AZ Caspi A. Impaired vestibulo-ocular reflex (VOR) in spinocerebellar ataxia type 3 (SCA3): bedside and search coil evaluation. J Vestib Res. 2014;24:351–5. https://doi.org/10.3233/VES-140527.
Luis L, Costa J, Munoz E, et al. Vestibulo-ocular reflex dynamics with head-impulses discriminates spinocerebellar ataxias types 1, 2 and 3 and Friedreich ataxia. J Vestib Res. 2016;26(3):327–34. https://doi.org/10.3233/VES-160579.
Zaltzman R, Sharony R, Klein C, Gordon CR. Spinocerebellar ataxia type 3 in Israel: phenotype and genotype of a Jew Yemenite subpopulation. J Neurol. 2016;263(11):2207–14. https://doi.org/10.1007/s00415-016-8251-8.
Geisinger D, Elyoseph Z, Zaltzman R, Mintz M, Gordon CR. Angular vestibulo ocular reflex loss with preserved saccular function in Machado-Joseph disease. J Neurol Sci. 2021;424:117393. https://doi.org/10.1016/J.JNS.2021.117393.
MacDougall HG, Weber KP, McGarvie LA, Halmagyi GM, Curthoys IS. The video head impulse test: diagnostic accuracy in peripheral vestibulopathy. Neurology. 2009;73(14):1134–41. https://doi.org/10.1212/WNL.0b013e3181bacf85.
Schmitz-Hübsch T, Du Montcel ST, Baliko L, Berciano J, Boesch S, Depondt C, Giunti P, Globas C, Infante J, Kang JS, Kremer B. Scale for the assessment and rating of ataxia. Neurology. 2006;66(11):1717–20. https://doi.org/10.1212/01.WNL.0000219042.60538.92.
Weyer A, Abele M, Schmitz-Hübsch T, et al. Reliability and validity of the scale for the assessment and rating of ataxia: a study in 64 ataxia patients. Mov Disord. 2007;22(11):1633–7. https://doi.org/10.1002/MDS.21544.
Maas R, Van Gaalen J, Klockgether T, Van de Warrenburg BPC. The preclinical stage of spinocerebellar ataxias. Neurology. 2015;85(1):96–103. https://doi.org/10.1212/WNL.0000000000001711.
Guan Q, Zhang L, Hong W, et al. Video Head Impulse Test for early diagnosis of vestibular neuritis among acute vertigo. Can J Neurol Sci. 2017;44(5):556–61. https://doi.org/10.1017/cjn.2017.202.
Elsherif M, Eldeeb M. Video head impulse test in bilateral vestibulopathy. Brazilian J Otorhinolaryngol (English Ed. Published online). 2020;88:181–6. https://doi.org/10.1016/J.BJORL.2020.05.014.
Jardim LB, Hauser L, Kieling C, et al. Progression rate of neurological deficits in a 10-year cohort of SCA3 patients. Cerebellum. 2010;9(3):419–28. https://doi.org/10.1007/s12311-010-0179-4.
de Oliveira CM, Leotti VB, Bolzan G, et al. Pre-ataxic changes of clinical scales and eye movement in Machado-Joseph disease: BIGPRO study. Mov Disord. 2021;36(4):985–94.
de Oliveira CM, Leotti VB, Cappelli AH, et al. Progression of clinical and eye movement markers in pre-ataxic carriers of Machado-Joseph disease. Mov Disord. 2022;38(1):26–34. https://doi.org/10.1002/mds.29226.
Gittis AH, du Lac S. Intrinsic and synaptic plasticity in the vestibular system. Curr Opin Neurobiol. 2006;16(4):385–90. https://doi.org/10.1016/J.CONB.2006.06.012.
Ashizawa T, Figueroa KP, Perlman SL, et al. Clinical characteristics of patients with spinocerebellar ataxias 1, 2, 3 and 6 in the US; a prospective observational study. Orphanet J Rare Dis. 2013;8(1):1–8. https://doi.org/10.1186/1750-1172-8-177.
Jacobi H, du Montcel ST, Bauer P, et al. Long-term disease progression in spinocerebellar ataxia types 1, 2, 3, and 6: a longitudinal cohort study. Lancet Neurol. 2015;14(11):1101–8. https://doi.org/10.1016/S1474-4422(15)00202-1.
Lin YC, Lee YC, Hsu TY, Liao YC, Soong BW. Comparable progression of spinocerebellar ataxias between Caucasians and Chinese. Park Relat Disord. 2019;62:156–62. https://doi.org/10.1016/j.parkreldis.2018.12.023.
Leotti VB, de Vries JJ, Oliveira CM, de Mattos EP, Te Meerman GJ, Brunt ER, Kampinga HH, Jardim LB, Verbeek DS. CAG repeat size influences the progression rate of spinocerebellar ataxia type 3. Ann Neurol. 2021;89(1):66–73. https://doi.org/10.1002/ana.25919.
Acknowledgements
The authors thank the patients and families that participated in the study.
Funding
This work was supported by the Israeli Chief Scientist Office, Ministry of Health (CSO MOH, IL) within the framework of the European-Latin America Consortium (EU-LAC) Health Joint Fund (grant # 3-000-14307). There is no further involvement of the funding body in this study.
Author information
Authors and Affiliations
Contributions
Zohar Elyoseph: conception and design of the study, acquisition and analysis of data, drafting a significant portion of the manuscript and figures.
Dario Geisinger: conception and design of the study, acquisition and analysis of data, drafting a significant portion of the manuscript.
Roy Zaltzman: conception and design of the study, acquisition and analysis of data, drafting a significant portion of the manuscript.
Matti Mintz: conception and design of the study, drafting a significant portion of the manuscript.
Carlos R. Gordon: conception and design of the study, acquisition and analysis of data, drafting a significant portion of the manuscript and figures.
Corresponding author
Ethics declarations
Ethical Approval
The protocol of the study was approved by the Ethics Committee (Institutional Review Board) of the Meir Medical Center, Kfar-Saba, Israel, and followed the tenets of the Declaration of Helsinki.
Competing Interests
The authors declare no competing interests.
Disclaimer
The funders had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Zohar Elyoseph and Dario Geisinger contributed equally to the manuscript.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Elyoseph, Z., Geisinger, D., Zaltzman, R. et al. Horizontal Vestibulo-Ocular Reflex Deficit as a Biomarker for Clinical Disease Onset, Severity, and Progression of Machado-Joseph Disease. Cerebellum (2023). https://doi.org/10.1007/s12311-023-01552-2
Accepted:
Published:
DOI: https://doi.org/10.1007/s12311-023-01552-2