Abstract
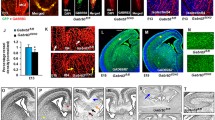
Intercellular influences are necessary for coordinated development and function of vascular and neural components in the brain. In the early postnatal period after birth, the mammalian cerebellum undergoes extensive morphogenesis — developing its characteristic lobules, organizing its diverse cell types into defined cellular layers, and establishing neural circuits that support cerebellar function, such as coordinated movement. In parallel, the cerebellar vasculature undergoes extensive postnatal growth and maturation, keeping pace with the expanding neural compartment. Endothelial deletion of Rbpj leads to neurovascular abnormalities in mice, including arteriovenous (AV) shunts that supplant capillaries and instead direct high-pressure/high-flow arterial blood directly to veins. Gross and histopathological cerebellar abnormalities, associated with these Rbpj-mediated brain AV malformations (AVMs), led to our hypothesis that early postnatal morphogenesis and lamination of cerebellum was perturbed in mice harboring endothelial Rbpj deficiency from birth. Here, we show that endothelial Rbpj-mutant mice developed enlarged vascular malformations on the cerebellar surface, by 2-week post-Rbpj deletion. In addition, outgrowth of cerebellar lobules was impaired through decreased cell proliferation, but not increased apoptosis, in the external granule layer. Molecular layer thickness was reduced, and the Purkinje layer was affected, by decreased Purkinje cell number, primary dendrite length, and dendritic arbor density. Endothelial deletion of Rbpj also led to impaired motor behaviors, consistent with abnormal cerebellar morphogenesis and lamination. Thus, our data suggest that Rbpj is required, in early postnatal vascular endothelium, to ensure proper cerebellar outgrowth, morphogenesis, and function in mice.






Similar content being viewed by others
References
White JJ, Sillitoe RV. Development of the cerebellum: from gene expression patterns to circuit maps: Development of cerebellum. Wiley Interdiscip Rev Dev Biol. 2013;2:149–64.
Ito M. Historical review of the significance of the cerebellum and the role of Purkinje cells in motor learning. Ann N Y Acad Sci. 2002;978:273–88.
Acker T, Beck H, Plate KH. Cell type specific expression of vascular endothelial growth factor and angiopoietin-1 and -2 suggests an important role of astrocytes in cerebellar vascularization. Mech Dev. 2001;108:45–57.
Sotelo C, Dusart I. Intrinsic versus extrinsic determinants during the development of Purkinje cell dendrites. Neuroscience. 2009;162:589–600.
Dusart I, Flamant F. Profound morphological and functional changes of rodent Purkinje cells between the first and the second postnatal weeks: a metamorphosis? Front Neuroanat [Internet]. 2012 [cited 2021 Dec 15];6. Available from: http://journal.frontiersin.org/article/https://doi.org/10.3389/fnana.2012.00011/abstract
Sudarov A, Joyner AL. Cerebellum morphogenesis: the foliation pattern is orchestrated by multi-cellular anchoring centers. Neural Develop. 2007;2:26.
Snapyan M, Lemasson M, Brill MS, Blais M, Massouh M, Ninkovic J, et al. Vasculature guides migrating neuronal precursors in the adult mammalian forebrain via brain-derived neurotrophic factor signaling. J Neurosci. 2009;29:4172–88.
Won C, Lin Z, Kumar TP, Li S, Ding L, Elkhal A, et al. Autonomous vascular networks synchronize GABA neuron migration in the embryonic forebrain. Nat Commun. 2013;4:2149.
Xi Y, Chen WJ, Deng JX, Cui ZJ, Liu HL, Yan MC, et al. Vasculature-guided neural migration in mouse cerebellum. Ital J Zool. 2015;82:15–24.
Paredes MF, James D, Gil-Perotin S, Kim H, Cotter JA, Ng C, et al. Extensive migration of young neurons into the infant human frontal lobe. Science. 2016;354:aaf7073.
Tsai H-H, Niu J, Munji R, Davalos D, Chang J, Zhang H, et al. Oligodendrocyte precursors migrate along vasculature in the developing nervous system. Science. 2016;351:379–84.
Tan X, Liu WA, Zhang X-J, Shi W, Ren S-Q, Li Z, et al. Vascular influence on ventral telencephalic progenitors and neocortical interneuron production. Dev Cell. 2016;36:624–38.
Daneman R, Zhou L, Agalliu D, Cahoy JD, Kaushal A, Barres BA. The mouse blood-brain barrier transcriptome: a new resource for understanding the development and function of brain endothelial cells. Ikezu T, editor. PLoS ONE. 2010;5:e13741.
Bjornsson CS, Apostolopoulou M, Tian Y, Temple S. It takes a village: constructing the neurogenic niche. Dev Cell. 2015;32:435–46.
Paredes I, Himmels P, Ruiz de Almodóvar C. Neurovascular communication during CNS development. Dev Cell. 2018;45:10–32.
Javaherian A, Kriegstein A. A stem cell niche for intermediate progenitor cells of the embryonic cortex. Cereb Cortex. 2009;19:i70–7.
Li S, Haigh K, Haigh JJ, Vasudevan A. Endothelial VEGF sculpts cortical cytoarchitecture. J Neurosci. 2013;33:14809–15.
Ottone C, Krusche B, Whitby A, Clements M, Quadrato G, Pitulescu ME, et al. Direct cell–cell contact with the vascular niche maintains quiescent neural stem cells. Nat Cell Biol. 2014;16:1045–56.
Licht T, Keshet E. The vascular niche in adult neurogenesis. Mech Dev. 2015;138:56–62.
Ramasamy SK, Kusumbe AP, Adams RH. Regulation of tissue morphogenesis by endothelial cell-derived signals. Trends Cell Biol. 2015;25:148–57.
Borggrefe T, Oswald F. The Notch signaling pathway: transcriptional regulation at Notch target genes. Cell Mol Life Sci. 2009;66:1631–46.
Fernández-Chacón M, García-González I, Mühleder S, Benedito R. Role of Notch in endothelial biology. Angiogenesis. 2021;24:237–50.
Krebs LT, Xue Y, Norton CR, Shutter JR, Maguire M, Sundberg JP, et al. Notch signaling is essential for vascular morphogenesis in mice. Genes Dev. 2000;14:1343–52.
Krebs LT, Shutter JR, Tanigaki K, Honjo T, Stark KL, Gridley T. Haploinsufficient lethality and formation of arteriovenous malformations in Notch pathway mutants. Genes Dev. 2004;18:2469–73.
Copeland JN, Feng Y, Neradugomma NK, Fields PE, Vivian JL. Notch signaling regulates remodeling and vessel diameter in the extraembryonic yolk sac. BMC Dev Biol. 2011;11:12.
Nielsen CM, Cuervo H, Ding VW, Kong Y, Huang EJ, Wang RA. Deletion of Rbpj from postnatal endothelium leads to abnormal arteriovenous shunting in mice. Development. 2014;141:3782–92.
Cuervo H, Nielsen CM, Simonetto DA, Ferrell L, Shah VH, Wang RA. Endothelial notch signaling is essential to prevent hepatic vascular malformations in mice: Notch signaling prevents hepatic vascular malformations in mice. Hepatology. 2016;64:1302–16.
Sörensen I, Adams RH, Gossler A. DLL1-mediated Notch activation regulates endothelial identity in mouse fetal arteries. Blood. 2009;113:5680–8.
Tanigaki K, Han H, Yamamoto N, Tashiro K, Ikegawa M, Kuroda K, et al. Notch–RBP-J signaling is involved in cell fate determination of marginal zone B cells. Nat Immunol. 2002;3:443–50.
Muzumdar MD, Tasic B, Miyamichi K, Li L, Luo L. A global double-fluorescent Cre reporter mouse. Genesis. 2007;45:593–605.
Hassell J, Hand AR. Tissue fixation with diimidoesters as an alternative to aldehydes I. Comparison of cross-linking and ultrastructure obtained with dimethylsuberimidate and glutaraldehyde. J Histochem Cytochem. 1974;22:223–39.
Feather-Schussler DN, Ferguson TS. A battery of motor tests in a neonatal mouse model of cerebral palsy. J Vis Exp. 2016;53569.
Corrales JD, Blaess S, Mahoney EM, Joyner AL. The level of sonic hedgehog signaling regulates the complexity of cerebellar foliation. Development. 2006;133:1811–21.
Wechsler-Reya RJ, Scott MP. Control of neuronal precursor proliferation in the cerebellum by sonic hedgehog. Neuron. 1999;22:103–14.
Sillitoe RV, Joyner AL. Morphology, molecular codes, and circuitry produce the three-dimensional complexity of the cerebellum. Annu Rev Cell Dev Biol. 2007;23:549–77.
Ilg W, Giese MA, Gizewski ER, Schoch B, Timmann D. The influence of focal cerebellar lesions on the control and adaptation of gait. Brain. 2008;131:2913–27.
Carrillo J, Nishiyama N, Nishiyama H. Dendritic translocation establishes the winner in cerebellar climbing fiber synapse elimination. J Neurosci. 2013;33:7641–53.
Szymczak M, Krupa P, Oszkinis G, Majchrzycki M. Gait pattern in patients with peripheral artery disease. BMC Geriatr. 2018;18:52.
de Laat KF, van Norden AGW, Gons RAR, van Oudheusden LJB, van Uden IWM, Bloem BR, et al. Gait in elderly with cerebral small vessel disease. Stroke. 2010;41:1652–8.
Krauss JK, Kiriyanthan GD, Borremans JJ. Cerebral arteriovenous malformations and movement disorders. Clin Neurol Neurosurg. 1999;101:92–9.
Sweeney MD, Ayyadurai S, Zlokovic BV. Pericytes of the neurovascular unit: key functions and signaling pathways. Nat Neurosci. 2016;19:771–83.
University of California. San Francisco Arteriovenous Malformation Study Project, Rodríguez-Hernández A, Kim H, Pourmohamad T, Young WL. Lawton MT Cerebellar arteriovenous malformations Neurosurgery. 2012;71:1111–24.
Acknowledgements
We thank Kayleigh Fanelli for assistance with confocal imaging; Ohio University IACUC and Laboratory for Animal Research for animal care; Ohio University Histopathology Core for access to cryostat; and Ohio University Neuroscience Program for access to confocal microscope.
Funding
This research was supported by Ohio University Honors Tutorial College Research Apprenticeship to A.D.C.; Ohio University Summer Neuroscience Undergraduate Research Fellowships to A.L.-B., S.S., and T.R.W.; and start-up funding from Ohio University College of Arts & Sciences to C.M.N.
Author information
Authors and Affiliations
Contributions
A.D.C. and C.M.N. conceptualized and designed experiments; A.D.C., S.S., J.L., A.L.-B., T.R.W., S.A., and C.M.N. performed experiments and analyzed data; and A.D.C. and C.M.N. wrote the manuscript.
Corresponding author
Ethics declarations
Competing Interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Chapman, A.D., Selhorst, S., LaComb, J. et al. Endothelial Rbpj Is Required for Cerebellar Morphogenesis and Motor Control in the Early Postnatal Mouse Brain. Cerebellum 22, 613–627 (2023). https://doi.org/10.1007/s12311-022-01429-w
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12311-022-01429-w