Abstract
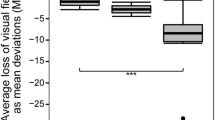
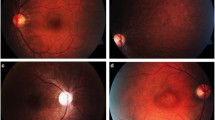
The aim of this study is to propose a classification system for the spinocerebellar ataxia type 7 retinal degeneration (SCA7-RD). Twenty patients with molecularly confirmed SCA7 underwent slit lamp examination, fundus photography, and optical coherence tomography (Spectralis®). Scale for the Assessment and Rating of Ataxia (SARA) and International Cooperative Ataxia Rating Scale (ICARS) were applied, and age, sex, age at symptom onset, and number of CAG expansions were recorded. After analyzing the ophthalmological findings in each participant, a panel of retinal disease experts created a qualitative classification system for SCA7-RD comprising four stages. We assessed the correlations of retinal degeneration severity with SARA and ICARS scores, number of CAG repeats in ATXN7 allele, and age at symptom onset. We graded retinal degeneration as stage 1 in nine participants, as stage 2 in five, and as stage 3 in six. No differences in age and visual symptoms duration were found between groups. SARA and ICARS scores correlated with the severity of SCA7-RD on the classification system (p = 0.024 and p = 0.014, respectively). After adjusting for disease duration, retinal disease stage association with SARA and ICARS scores remained significant (ANCOVA, p < 0.05). The classification system for SCA7-RD was able to characterize different disease stages representing the landmarks in the cone–rod dystrophy natural history. Neurodegeneration appears to occur in parallel in the cerebellum and in the visual pathway. We conclude that retinal degeneration in SCA7 is a potential biomarker of the neurological phenotype severity.

Similar content being viewed by others
References
Schols L, Bauer P, Schmidt T, Schulte T, Riess O. Autosomal dominant cerebellar ataxias: clinical features, genetics and pathogenesis. Lancet Neurol. 2004;3(5):291–304.
Martin JJ. Spinocerebellar ataxia type 7. In: Subramony SH, Durr A, editors. Handbook of clinical neurology, vol. 103; 2012. p. 475–91.
Albuquerque MVC, Pedroso JL, Braga-Neto P, Barsottini OG. Phenotype and early onset ataxia symptoms in spinocerebellar ataxia type 7: comparison and correlation with other spinocerebellar ataxias. Arq Neuropsiquiatr. 2015;73(1):18–21.
David G, Durr A, Stevanin G, et al. Molecular and clinical correlations in autosomal dominant cerebellar ataxia with progressive macular dystrophy (SCA 7). Hum Mol Genet. 1998;7(2):165–70.
Aleman TS, Cideciyan AV, Volpe NJ, Stevanin G, Brice A, Jacobson SG. Spinocerebellar ataxia type 7 shows a cone-rod dystrophy phenotype. Exp Eye Res. 2002;74(6):737–45.
Thurtell MJ, Fraser JA, Bala E, Tomsak RL, Biousse V, Leigh RJ, et al. Two patients with spinocerebellar ataxia type 7 presenting with profound binocular visual loss yet minimal ophthalmoscopic findings. J Neuroophthalmol. 2009;29(3):187–91.
Anh JK, Seo JM, Chung H, et al. Anatomical and functional characteristics in atrophic maculopathy associated with spinocerebellar ataxia type 7. Am J Ophthalmol. 2005;139(5):923–5.
Horton LC, Frosch MP, Vangel MG, Weigel-DiFranco C, Berson EL, Schmahmann JD. Spinocerebellar ataxia type 7: clinical course, phenotype-genotype correlations, and neuropathology. Cerebellum. 2013;12(2):176–93.
Hugosson T, Granse L, Ponjavic V, Andréasson S. Macular dysfunction and morphology in spinocerebellar ataxia type 7. Ophthalmic Genet. 2009;30(1):1–6.
Miller RC, Tewari A, Miller JA, Garbern J, Van Stavern G. Neuro-ophthalmologic features of spinocerebellar ataxia type 7. J Neuroophthalmol. 2009;29(3):180–6.
Manrique RK, Noval S, Aguilar-Amat MJ, Arpa J, Rosa I, Contreras I. Ophthalmic features of spinocerebellar ataxia type 7. J Neuroophthalmol. 2009;29(3):174–9.
Watkins WM, Schoenberger SD, Lavin P, Agarwal A. Circumscribed outer foveolar defects in spinocerebellar ataxia type 7. Retin Cases Brief Rep. 2013;7(3):294–6.
Levinson JD, Yan J, Lambert SR, Shankar SP. Multimodal imaging of a family with spinocerebellar ataxia type 7 demonstrating phenotypic variation and progression of retinal degeneration. Retin Cases Brief Rep. 2016;10(3):267–72.
Yip G, Henao M, Huang LL. Retinal manifestations of spinocerebellar ataxia type 7 in two consecutive generations. Retin Cases Brief Rep. 2017;11(1):86–9.
Campos-Romo A, Graue-Hernandez EO, Pedro-Aguilar L, Hernandez-Camarena JC, Rivera-De la Parra D, Galvez V, et al. Ophthalmic features of spinocerebellar ataxia type 7. Eye. 2018;32(1):120–7.
Staurenghi G, Sadda S, Chakravarthy U, Spaide RF. Proposed lexicon for anatomic landmarks in normal posterior segment spectral-domain optical coherence tomography. Ophthalmology. 2014;121(8):1572–8.
Kersten HM, Roxburgh RH, Danesh-Meyer HV. Ophthalmic manifestations of inherited neurodegenerative disorders. Nat Rev Neurol. 2014;10:349–62.
Rezende Filho FM, Parkinson MH, Pedroso JL, Poh R, Faber I, Lourenço CM, et al. Clinical, ophthalmological, imaging and genetic features in Brazilian patients with ARSACS. Parkinsonism Relat Disord. 2019;62:148–55.
Freitas JL, Rezende Filho FM, Sallum JMF, França MC Jr, Pedroso JL, Barsottini OGP. Ophthalmological changes in hereditary spastic paraplegia and other genetic diseases with spastic paraplegia. J Neurol Sci. 2020;409:116620.
Britze J, Frederiksen JL. Optical coherence tomography in multiple sclerosis. Eye (Lond). 2018;32(5):884–8.
Lee JY, Ahn J, Kim TW, Jeon BS. Optical coherence tomography in Parkinson’s disease: is the retina a biomarker? J Parkinsons Dis. 2014;4(2):197–204.
Doustar J, Torbati T, Black KL, Koronyo Y, Koronyo-Hamaoui M. Optical coherence tomography in Alzheimer’s disease and other neurodegenerative diseases. Front Neurol. 2017;19(8):701.
Azevedo PB, Rocha AG, Keim LMN, Lavinsky D, Furtado GV, de Mattos EP, et al. Ophthalmological and neurologic manifestations in pre-clinical and clinical phases of spinocerebellar ataxia type 7. Cerebellum. 2019;18(3):388–96.
Hernandez-Castillo CR, Vaca-Palomares I, Barrios F, Martinez L, Boll MC, Fernandez-Ruiz J. Ataxia severity correlates with white matter degeneration in spinocerebellar ataxia type 7. AJNR Am J Neuroradiol. 2016;37(11):2050–4.
Hernandez-Castillo CR, Galvez V, Diaz R, Fernandez-Ruiz J. Specific cerebellar and cortical degeneration correlates with ataxia severity in spinocerebellar ataxia type 7. Brain Imaging Behav. 2016;10(1):252–7.
Velázquez-Pérez L, Cerecedo-Zapata CM, Hernández-Hernández O, Martínez-Cruz E, Tapia-Guerrero YS, González-Piña R, et al. A comprehensive clinical and genetic study of a large Mexican population with spinocerebellar ataxia type 7. Neurogenetics. 2014;16(1):11–21.
Salas-Vargas J, Mancera-Gervacio J, Velázquez-Pérez L, Rodrígez-Labrada R, Martínez-Cruz E, Magaña JJ, et al. Spinocerebellar ataxia type 7: a neurodegenerative disorder with peripheral neuropathy. Eur Neurol. 2015;73(3–4):173–8.
Alvarez G, Rey A, Sanchez-Dalmau FB, Muñoz E, Ríos J, Adán A. Optical coherence tomography findings in spinocerebellar ataxia-3. Eye. 2013;27(12):1376–81.
Fortuna F, Barboni P, Liguori R, Valentino ML, Savini G, Gellera C, et al. Visual system involvement in patients with Friedreich’s ataxia. Brain. 2009;132:116–23.
Chen S, Peng GH, Wang X, Smith AC, Grote SK, Sopher BL, et al. Interference of Crx-dependent transcription by ataxin-7 involves interaction between the glutamine regions and requires the ataxin-7 carboxy-terminal region for nuclear localization. Hum Mol Genet. 2004;13(1):53–67.
Karam A, Trottier Y. Molecular mechanisms and therapeutic strategies in spinocerebellar ataxia type 7. Adv Exp Med Biol. 2018;1049:197–218.
Ramachandran PS, Bhattarai S, Singh P, Boudreau RL, Thompson S, Laspada AR, et al. RNA interference-based therapy for spinocerebellar ataxia type 7 retinal degeneration. PLoS One. 2014;9(4):e95362.
Niu C, Prakash TP, Kim A, Quach JL, Huryn LA, Yang Y, et al. 2 Antisense oligonucleotides targeting mutant Ataxin-7 restore visual function in a mouse model of spinocerebellar ataxia type 7. Sci Transl Med. 2018;10(465):eaap8677.
Roosing S, Thiadens AA, Hoyng CB, Klaver CC, den Hollander AI, Cremers FP. Causes and consequences of inherited cone disorders. Prog Retin Eye Res. 2014;42:1–26.
Michaelides M, Hardcastle AJ, Hunt DM, Moore AT. Progressive cone and cone-rod dystrophies: phenotypes and underlying molecular genetic basis. Surv Ophthalmol. 2006;51(3):232–58.
Thiadens AA, Phan TM, Zekveld-Vroon RC, Leroy BP, van den Born L, Hoyng CB, et al. Clinical course, genetic etiology, and visual outcome in cone and cone-rod dystrophy. Ophthalmology. 2012;119(4):819–26.
La Spada AR. Spinocerebellar ataxia type 7. [updated 2020 Jul 23]. In: Adam MP, Ardinger HH, Pagon RA, et al., editors. GeneReviews® [Internet]. Seattle: University of Washington, Seattle; 1998. p. 1993-2020.
Johansson J, Forsgren L, Sandgren O, Brice A, Holmgren G, Holmberg M. Expanded CAG repeats in Swedish spinocerebellar ataxia type 7 (SCA7) patients: effect of CAG repeat length on the clinical manifestation. Hum Mol Genet. 1998;7(2):171–6.
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Material preparation, data collection, and analysis were performed by Bruna Ferraço Marianelli, Flávio Moura Rezende Filho, Mariana Vallim Salles, João Brainer Clares de Andrade, José Luiz Pedroso, Juliana Maria Ferraz Sallum, and Orlando G. Barsottini. The first draft of the manuscript was written by Bruna Ferraço Marianelli and Flávio Moura Rezende Filho, and all authors commented on previous versions of the manuscript and added important contributions on the review process. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics Approval and Consent to Participate
The authors declare that this study was approved by the Research Ethics Committee, and all participants provided informed consent. Project number and institution responsible for the approval of the Research Ethics Committee: Ethic Committee of Federal University of São Paulo (number of Register: 54042316.7.0000.5505).
Consent for Publication
The authors declare that the informed consent of all participants was obtained. All individuals included in this study allowed publication of clinical data, including examination images, with the guarantee that their identity would be protected.
Conflict of Interest
We declare no conflicts of interest and no financial support for this work.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Marianelli, B.F., Filho, F.M.R., Salles, M.V. et al. A Proposal for Classification of Retinal Degeneration in Spinocerebellar Ataxia Type 7. Cerebellum 20, 384–391 (2021). https://doi.org/10.1007/s12311-020-01215-6
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12311-020-01215-6