Abstract
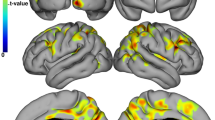
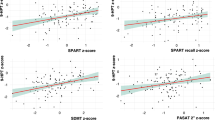
Both histological and neuroimaging studies highlight the role of the cerebellum in multiple sclerosis (MS). There is at least some evidence for associations of cerebellar gray matter (GM) loss with motor and cognitive ability. We therefore correlated motor and cognitive ability scores (the multiple sclerosis functional composite MSFC) with regional cerebellar GM volumes. We used voxel-based morphometry (VBM) to assess the regional GM volume loss in a cohort of 45 MS patients. For the regression analysis, we used the clinical subscores of the multiple sclerosis functional composite (25-ft walk test (T25FW), nine-hole peg test (9HPT), paced auditory serial addition task (PASAT)). Decreased GM in distinct cerebellar areas was associated with different subscores of the MSFC in Larsell’s lobule VI with the T25FW (t = 5.16), in lobule IX with the 9HPT (t = 3.95), and in lobule IX with the PASAT (t = 4.81). Regional volume decrease in distinct cerebellar areas involved in motor and cognitive domains were associated with clinical impairment in these fields. Our data confirm the relationship between cerebellar GM volume loss and disability, extending the knowledge in the functional neuroanatomical perspective.


Similar content being viewed by others
References
Compston A, Coles A. Multiple sclerosis. Lancet. 2008;372:1502–17.
Filippi M, Rocca MA, Barkhof F, et al. Association between pathological and MRI findings in multiple sclerosis. Lancet Neurol. 2012;11:349–60.
Kutzelnigg A, Lucchinetti CF, Stadelmann C, et al. Cortical demyelination and diffuse white matter injury in multiple sclerosis. Brain. 2005;128:2705–12.
Gilmore CP, Donaldson I, Bo L, Owens T, Lowe J, Evangelou N. Regional variations in the extent and pattern of grey matter demyelination in multiple sclerosis: a comparison between the cerebral cortex, cerebellar cortex, deep grey matter nuclei and the spinal cord. J Neurol Neurosurg Psychiatry. 2009;80:182–7.
Deppe M, Tabelow K, Kramer J, et al. Evidence for early, non-lesional cerebellar damage in patients with multiple sclerosis: DTI measures correlate with disability, atrophy, and disease duration. Mult Scler. 2015;
Weier K, Till C, Fonov V, et al. Contribution of the cerebellum to cognitive performance in children and adolescents with multiple sclerosis. Mult Scler. 2015;
Thurling M, Kahl F, Maderwald S, et al. Cerebellar cortex and cerebellar nuclei are concomitantly activated during eyeblink conditioning: a 7T fMRI study in humans. J Neurosci. 2015;35:1228–39.
Koziol LF, Budding D, Andreasen N, et al. Consensus paper: the cerebellum's role in movement and cognition. Cerebellum. 2014;13:151–77.
Stoodley CJ, Schmahmann JD. Functional topography in the human cerebellum: a meta-analysis of neuroimaging studies. NeuroImage. 2009;44:489–501.
Stoodley CJ, Valera EM, Schmahmann JD. Functional topography of the cerebellum for motor and cognitive tasks: an fMRI study. NeuroImage. 2012;59:1560–70.
Fischer JS, Rudick RA, Cutter GR, Reingold SC. The multiple sclerosis functional composite measure (MSFC): an integrated approach to MS clinical outcome assessment. National MS Society clinical outcomes assessment task force. Mult Scler. 1999;5:244–50.
Polman CH, Rudick RA. The multiple sclerosis functional composite: a clinically meaningful measure of disability. Neurology. 2010;74(Suppl 3):S8–15.
Ashburner J, Friston KJ. Voxel-based morphometry—the methods. NeuroImage. 2000;11:805–21.
Polman CH, Reingold SC, Banwell B, et al. Diagnostic criteria for multiple sclerosis: 2010 revisions to the McDonald criteria. Ann Neurol. 2011;69:292–302.
Kurtzke JF. Rating neurologic impairment in multiple sclerosis: an expanded disability status scale (EDSS). Neurology. 1983;33:1444–52.
Beck AT, Steer RA. Beck depression inventory (BDI). San Antonio: The Psychological Corporation Inc.; 1987.
Krupp LB, LaRocca NG, Muir-Nash J, Steinberg AD. The fatigue severity scale. Application to patients with multiple sclerosis and systemic lupus erythematosus. Arch Neurol. 1989;46:1121–3.
Ceccarelli A, Jackson JS, Tauhid S, et al. The impact of lesion in-painting and registration methods on voxel-based morphometry in detecting regional cerebral gray matter atrophy in multiple sclerosis. AJNR Am J Neuroradiol. 2012;33:1579–85.
Grothe M, Lotze M, Langner S, Dressel A. The role of global and regional gray matter volume decrease in multiple sclerosis. J Neurol. 2016;263:1137–45.
Schmidt P, Gaser C, Arsic M, et al. An automated tool for detection of FLAIR-hyperintense white-matter lesions in multiple sclerosis. NeuroImage. 2012;59:3774–83.
Ashburner J. A fast diffeomorphic image registration algorithm. NeuroImage. 2007;38:95–113.
Mechelli A, Friston KJ, Frackowiak RS, Price CJ. Structural covariance in the human cortex. J Neurosci. 2005;25:8303–10.
Tzourio-Mazoyer N, Landeau B, Papathanassiou D, et al. Automated anatomical labeling of activations in SPM using a macroscopic anatomical parcellation of the MNI MRI single-subject brain. NeuroImage. 2002;15:273–89.
Weier K, Banwell B, Cerasa A, et al. The role of the cerebellum in multiple sclerosis. Cerebellum. 2015;14:364–74.
Kutzelnigg A, Faber-Rod JC, Bauer J, et al. Widespread demyelination in the cerebellar cortex in multiple sclerosis. Brain Pathol. 2007;17:38–44.
Audoin B, Zaaraoui W, Reuter F, et al. Atrophy mainly affects the limbic system and the deep grey matter at the first stage of multiple sclerosis. J Neurol Neurosurg Psychiatry. 2010;81:690–5.
Anderson VM, Fisniku LK, Altmann DR, Thompson AJ, Miller DH. MRI measures show significant cerebellar gray matter volume loss in multiple sclerosis and are associated with cerebellar dysfunction. Mult Scler. 2009;15:811–7.
Moroso A, Ruet A, Lamargue-Hamel D, et al. Posterior lobules of the cerebellum and information processing speed at various stages of multiple sclerosis. J Neurol Neurosurg Psychiatry. 2017;88:146–51.
D'Ambrosio A, Pagani E, Riccitelli GC, et al. Cerebellar contribution to motor and cognitive performance in multiple sclerosis: an MRI sub-regional volumetric analysis. Mult Scler. 2016;1352458516674567
Manni E, Petrosini L. A century of cerebellar somatotopy: a debated representation. Nat Rev Neurosci. 2004;5:241–9.
Cutter GR, Baier ML, Rudick RA, et al. Development of a multiple sclerosis functional composite as a clinical trial outcome measure. Brain. 1999;122(Pt 5):871–82.
Sbardella E, Petsas N, Tona F, et al. Assessing the correlation between grey and white matter damage with motor and cognitive impairment in multiple sclerosis patients. PLoS One. 2013;8:e63250.
Audoin B, Ibarrola D, Ranjeva JP, et al. Compensatory cortical activation observed by fMRI during a cognitive task at the earliest stage of MS. Hum Brain Mapp. 2003;20:51–8.
Valentino P, Cerasa A, Chiriaco C, et al. Cognitive deficits in multiple sclerosis patients with cerebellar symptoms. Mult Scler. 2009;15:854–9.
Moroso A, Ruet A, Deloire M, et al. Cerebellar assessment in early multiple sclerosis. Cerebellum. 2017;16:607–11.
Ruet A, Hamel D, Deloire MS, Charre-Morin J, Saubusse A, Brochet B. Information processing speed impairment and cerebellar dysfunction in relapsing-remitting multiple sclerosis. J Neurol Sci. 2014;347:246–50.
Archibald CJ, Wei X, Scott JN, et al. Posterior fossa lesion volume and slowed information processing in multiple sclerosis. Brain. 2004;127:1526–34.
Cerasa A, Valentino P, Chiriaco C, et al. MR imaging and cognitive correlates of relapsing-remitting multiple sclerosis patients with cerebellar symptoms. J Neurol. 2013;260:1358–66.
Weier K, Penner IK, Magon S, et al. Cerebellar abnormalities contribute to disability including cognitive impairment in multiple sclerosis. PLoS One. 2014;9:e86916.
Diedrichsen J. A spatially unbiased atlas template of the human cerebellum. NeuroImage. 2006;33:127–38.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The study was approved by the local ethical committees and written informed consent from each subject was obtained prior to their enrolment.
Conflict of Interests
M. Grothe has received travel reimbursement from Novartis Pharma, Teva, and BiogenIdec and research grants from the Federal Ministry for Research and Education in Germany.
M. Lotze has received research grants from the German Research Foundation and the Federal Ministry for Research and Education in Germany.
S. Langner received institutional support from the University of Greifswald for investigator initiated studies.
A. Dressel has received research grants, speaker and consulting honoraria as well as travel reimbursement from Novartis Pharma, Bayer Schering, Teva, Sanofi Aventis, Genzyme, Merck Serono, and BiogenIdec.
Rights and permissions
About this article
Cite this article
Grothe, M., Lotze, M., Langner, S. et al. Impairments in Walking Ability, Dexterity, and Cognitive Function in Multiple Sclerosis Are Associated with Different Regional Cerebellar Gray Matter Loss. Cerebellum 16, 945–950 (2017). https://doi.org/10.1007/s12311-017-0871-8
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12311-017-0871-8