Abstract
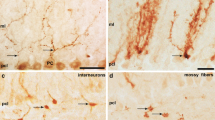
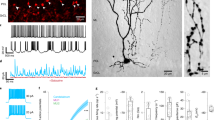
The cerebellum is organized into a map of zones that is manifested in various ways according to gene expression, anatomical connectivity, neuronal firing properties, behavioral specificity, and susceptibility to disease. At the center of every zone is the Purkinje cell, the principal cell type of the cerebellum and sole output of the cerebellar cortex. During development, Purkinje cells are thought to coordinate the zonal patterning of all other cell types. However, the morphogenetic mechanism that mediates the interaction between Purkinje cells and afferent fibers remains unclear. To address this problem in vivo, I took advantage of a rapid fluorescent-based transynaptic tracing approach to determine the nature of mossy fiber to Purkinje cell connectivity during early postnatal development, a period when the afferent map is assembling into clear-cut zonal circuits. By injecting WGA-Alexa 555 into the lower thoracic-upper lumber spinal cord, I found that spinocerebellar mossy fibers transynaptically transfer tracer into zones of Purkinje cells that are directly adjacent to the fibers. The traced Purkinje cell zones formed a zebrin-like pattern that was defined by the expression of neurofilament heavy chain (NFH), a marker of zones in the postnatal developing cerebellum. These results suggest that Purkinje cells generate the zonal circuit map by using molecular cues, neuronal activity, and synaptic contact.

Similar content being viewed by others
References
White JJ, Sillitoe RV. Development of the cerebellum: from gene expression patterns to circuit maps. Wiley Interdiscip Rev Dev Biol. 2013;2:149–64.
Hashimoto M, Mikoshiba K. Mediolateral compartmentalization of the cerebellum is determined on the “birth date” of Purkinje cells. J Neurosci. 2003;23:11342–51.
Sudarov A, Turnbull RK, Kim EJ, Lebel-Potter M, Guillemot F, Joyner AL. Ascl1 genetics reveals insights into cerebellum local circuit assembly. J Neurosci. 2011;31:11055–69.
Croci L, Chung SH, Masserdotti G, Gianola S, Bizzoca A, Gennarini G, Corradi A, Rossi F, Hawkes R, Consalez GG. A key role for the HLH transcription factor EBF2COE2, O/E-3 in Purkinje neuron migration and cerebellar cortical topography. Development. 2006;133:2719–29.
Millen K, Hui C, Joyner A. A role for En-2 and other murine homologues of Drosophila segment polarity genes in regulating positional information in the developing cerebellum. Development. 1995;121:3935–45.
Larouche M, Hawkes R. From clusters to stripes: the developmental origins of adult cerebellar compartmentation. Cerebellum. 2006;5:77–88.
Wassef M, Zanetta JP, Brehier A, Sotelo C. Transient biochemical compartmentalization of Purkinje cells during early cerebellar development. Dev Biol. 1985;111:129–37.
Brochu G, Maler L, Hawkes R. Zebrin II: a polypeptide antigen expressed selectively by Purkinje cells reveals compartments in rat and fish cerebellum. J Comp Neurol. 1990;291:538–52.
Marzban H, Chung S, Watanabe M, Hawkes R. Phospholipase Cbeta4 expression reveals the continuity of cerebellar topography through development. J Comp Neurol. 2007;502:857–71.
Fujita H, Morita N, Furuichi T, Sugihara I. Clustered fine compartmentalization of the mouse embryonic cerebellar cortex and its rearrangement into the postnatal striped configuration. J Neurosci. 2012;32:15688–703.
White JJ, Arancillo M, Stay TL, George-Jones NA, Levy SL, Heck DH, Sillitoe RV. Cerebellar zonal patterning relies on Purkinje cell neurotransmission. J Neurosci. 2014;34:8231–45.
Sotelo C. Cellular and genetic regulation of the development of the cerebellar system. Prog Neurobiol. 2004;72:295–339.
Apps R, Hawkes R. Cerebellar cortical organization: a one-map hypothesis. Nat Rev Neurosci. 2009;10:670–81.
Sillitoe RV, Vogel MW, Joyner AL. Engrailed homeobox genes regulate establishment of the cerebellar afferent circuit map. J Neurosci. 2010;30:10015–24.
Kalinovsky A, Boukhtouche F, Blazeski R, Bornmann C, Suzuki N, Mason CA, Scheiffele P. Development of axon-target specificity of ponto-cerebellar afferents. PLoS Biol. 2011;9:e1001013.
Reeber SL, Gebre SA, Sillitoe RV. Fluorescence mapping of afferent topography in three dimensions. Brain Struct Funct. 2011;216:159–69.
White JJ, Sillitoe RV. Postnatal development of cerebellar zones revealed by neurofilament heavy chain protein expression. Front Neuroanat. 2013;7:9.
Goshgarian HG, Buttry JL. The pattern and extent of retrograde transsynaptic transport of WGA-Alexa 488 in the phrenic motor system is dependent upon the site of application. J Neurosci Methods. 2014;222:156–64.
Takeda T, Maekawa K. Transient direct connection of vestibular mossy fibers to the vestibulocerebellar Purkinje cells in early postnatal development of kittens. Neuroscience. 1989;32:99–111.
Tolbert DL, Pittman T, Alisky JM, Clark BR. Chronic NMDA receptor blockade or muscimol inhibition of cerebellar cortical neuronal activity alters the development of spinocerebellar afferent topography. Dev Brain Res. 1994;80:268–74.
Acknowledgements
This work was supported by funds from Baylor College of Medicine and Texas Children’s Hospital. R.V.S. received support from The Bachmann-Strauss Dystonia and Parkinson Foundation, Inc., The Caroline Wiess Law Fund for Research in Molecular Medicine, BCM IDDRC 1U54HD083092, National Center For Research Resources C06RR029965, The Mrs. Clifford Elder White Graham Endowed Research Fund, and the National Institutes of Neurological Disorders and Stroke 1R01NS089664. The BCM IDDRC Neuropathology Core performed a portion of the immunohistochemistry and histology experiments. The content is solely the responsibility of the author and does not necessarily represent the official views of the National Center For Research Resources or the National Institutes of Health (NIH).
Conflicts of Interest
The author declares that he has no competing interests.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Sillitoe, R.V. Mossy Fibers Terminate Directly Within Purkinje Cell Zones During Mouse Development. Cerebellum 15, 14–17 (2016). https://doi.org/10.1007/s12311-015-0712-6
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12311-015-0712-6