Abstract
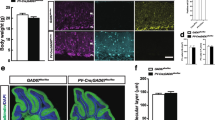
Excitatory amino acid transporter 4 (EAAT4) is believed to be critical to the synaptic activity of cerebellar Purkinje cells by limiting extracellular glutamate concentrations and facilitating the induction of long-term depression. However, the modulation of EAAT4 expression has not been elucidated. It has been shown that Ras homolog enriched in brain (Rheb)/mammalian target of rapamycin (mTOR) signaling plays essential roles in the regulation of protein translation, cell size, and cell growth. In addition, we previously found that a cascade including mTOR suppression and Akt activation induces increased expression of EAAT2 in astrocytes. In the present work, we explored whether Rheb/mTOR signaling is involved in the regulation of EAAT4 expression using conditional Rheb1 knockout mice. Our results demonstrated that Rheb1 deficiency resulted in the downregulation of EAAT4 expression, as well as decreased activity of mTOR and increased activity of Akt. The downregulation of EAAT4 was also confirmed by reduced EAAT4 currents and slowed kinetics of α-amino-3-hydroxy-5-methylisoxazole-4-propionic acid receptor–mediated currents. On the other hand, conditional knockout of Rheb1 did not alter the morphology of Purkinje cell layer and the number of Purkinje cells. Overall, our findings suggest that small GTPase Rheb1 is a modulator in the expression of EAAT4 in Purkinje cells.




Similar content being viewed by others
Abbreviations
- AMPA:
-
α-Amino-3-hydroxy-5-methylisoxazole-4-propionic acid
- EAAT:
-
Excitatory amino acid transporter
- EPSC:
-
Excitatory postsynaptic current
- GAPDH:
-
Glyceraldehyde 3-phosphate dehydrogenase
- GTRAP48:
-
Glutamate transporter-4-associated protein
- LTD:
-
Long-term depression
- mGluR:
-
Metabotropic glutamate receptors
- mTOR:
-
Mammalian target of rapamycin
- mTORC1:
-
mTOR complex 1
- NF-кB:
-
Nuclear factor-кB
- PSD95:
-
Postsynaptic density protein 95
- Rheb:
-
Ras homolog enriched in brain
- TSC:
-
Tuberous sclerosis complex
References
Billups B, Rossi D, Oshima T, Warr O, Takahashi M, Sarantis M, et al. Physiological and pathological operation of glutamate transporters. Prog Brain Res. 1998;116:45–57.
Yi JH, Hazell AS. Excitotoxic mechanisms and the role of astrocytic glutamate transporters in traumatic brain injury. Neurochem Int. 2006;48:394–403.
Huang YH, Bergles DE. Glutamate transporters bring competition to the synapse. Curr Opin Neurobiol. 2004;14:346–52.
Halassa MM, Fellin T, Haydon PG. The tripartite synapse: roles for gliotransmission in health and disease. Trends Mol Med. 2007;13:54–63.
Seal RP, Amara SG. Excitatory amino acid transporters: a family in flux. Ann Rev Pharmacol Toxicol. 1999;39:431–56.
Dehnes Y, Chaudhry FA, Ullensvang K, Lehre KP, Storm-Mathisen J, Danbolt NC. The glutamate transporter EAAT4 in rat cerebellar Purkinje cells: a glutamate-gated chloride channel concentrated near the synapse in parts of the dendritic membrane facing astroglia. J Neurosci. 1998;18:3606–19.
Fairman WA, Sonders MS, Murdoch GH, Amara SG. Arachidonic acid elicits a substrate-gated proton current associated with the glutamate transporter EAAT4. Nat Neurosci. 1998;1:105–13.
Massie A, Cnops L, Smolders I, McCullumsmith R, Kooijman R, Kwak S, et al. High-affinity Na+/K+-dependent glutamate transporter EAAT4 is expressed throughout the rat fore- and midbrain. J Comp Neurol. 2008;511:155–72.
Su LD, Shen Y. Blockade of glutamate transporters facilitates cerebellar synaptic long-term depression. Neuroreport. 2009;20:502–7.
Brasnjo G, Otis TS. Neuronal glutamate transporters control activation of postsynaptic metabotropic glutamate receptors and influence cerebellar long-term depression. Neuron. 2001;31:607–16.
Aspuria PJ, Tamanoi F. The Rheb family of GTP-binding proteins. Cell Signal. 2004;16:1105–12.
Inoki K, Li Y, Xu T, Guan KL. Rheb GTPase is a direct target of TSC2 GAP activity and regulates mTOR signaling. Genes Dev. 2003;17:1829–34.
Zhang Y, Gao X, Saucedo LJ, Ru B, Edgar BA, Pan D. Rheb is a direct target of the tuberous sclerosis tumour suppressor proteins. Nat Cell Biol. 2003;5:578–81.
Manning BD, Cantley LC. Rheb fills a GAP between TSC and TOR. Trends Biochem Sci. 2003;28:573–6.
Stocker H, Radimerski T, Schindelholz B, Wittwer F, Belawat P, Daram P, et al. Rheb is an essential regulator of S6K in controlling cell growth in Drosophila. Nat Cell Biol. 2003;5:559–65.
Ji YF, Zhou L, Xie YJ, Xu SM, Zhu J, Teng P, et al. Upregulation of glutamate transporter GLT-1 by mTOR-Akt-NF-κB cascade in astrocytic oxygen-glucose deprivation. Glia. 2013;61:1959–75.
Zou J, Zhou L, Du XX, Ji Y, Xu J, Tian J, et al. Rheb1 is required for mTORC1 and myelination in postnatal brain development. Dev Cell. 2011;20:97–108.
Barski JJ, Dethleffsen K, Meyer M. Cre recombinase expression in cerebellar Purkinje cells. Genesis. 2000;28:93–8.
Wu ZY, Zhu LJ, Zou N, Bombek LK, Shao CY, Wang N, et al. AMPA receptors regulate exocytosis and insulin release in pancreatic β cells. Traffic. 2012;13:1124–39.
Fukata Y, Lovero KL, Iwanaga T, Watanabe A, Yokoi N, Tabuchi K, et al. Disruption of LGI1-linked synaptic complex causes abnormal synaptic transmission and epilepsy. Proc Natl Acad Sci U S A. 2010;107:3799–804.
Shao CY, Zhu J, Xie YJ, Wang Z, Wang YN, Wang Y, et al. Distinct functions of nuclear distribution proteins LIS1, Ndel1 and NudCL in regulating axonal mitochondrial transport. Traffic. 2013;14:785–97.
Xie YJ, Zhou L, Jiang N, Zhang N, Zou N, Zhou L, et al. Essential roles of leucine-rich glioma inactivated 1 in the development of embryonic and postnatal cerebellum. Sci Rep. 2015;5:7827.
Wang DJ, Su LD, Wang YN, Yang D, Sun CL, Zhou L, et al. Long-term potentiation at cerebellar parallel fiber-Purkinje cell synapses requires presynaptic and postsynaptic signaling cascades. J Neurosci. 2014;34:2355–64.
Jackson M, Song W, Liu MY, Jin L, Dykes-Hoberg M, Lin CI, et al. Modulation of the neuronal glutamate transporter EAAT4 by two interacting proteins. Nature. 2001;410:89–93.
Harrington LS, Findlay GM, Gray A, Tolkacheva T, Wigfield S, Rebholz H, et al. The TSC1-2 tumor suppressor controls insulin-PI3K signaling via regulation of IRS proteins. J Cell Biol. 2004;166:213–23.
Shah OJ, Hunter T. Critical role of T-loop and H-motif phosphorylation in the regulation of S6 kinase 1 by the tuberous sclerosis complex. J Biol Chem. 2004;279:20816–23.
O’Reilly KE, Rojo F, She QB, Solit D, Mills GB, Smith D, et al. mTOR inhibition induces upstream receptor tyrosine kinase signaling and activates Akt. Cancer Res. 2006;66:1500–8.
Sarbassov DD, Ali SM, Sengupta S, Sheen JH, Hsu PP, Bagley AF, et al. Prolonged rapamycin treatment inhibits mTORC2 assembly and Akt/PKB. Mol Cell. 2006;22:159–68.
Sarbassov DD, Guertin DA, Ali SM, Sabatini DM. Phosphorylation and regulation of Akt/PKB by the rictor-mTOR complex. Science. 2005;307:1098–101.
Sun SY, Rosenberg LM, Wang X, Zhou Z, Yue P, Fu H, et al. Activation of Akt and eIF4E survival pathways by rapamycin-mediated mammalian target of rapamycin inhibition. Cancer Res. 2005;65:7052–8.
Shen Y, Linden DJ. Long-term potentiation of neuronal glutamate transporters. Neuron. 2005;46:715–22.
Shimamoto K, Lebrun B, Yasuda-Kamatani Y, Sakaitani M, Shigeri Y, Yumoto N, et al. DL-threo-beta-benzyloxyaspartate, a potent blocker of excitatory amino acid transporters. Mol Pharmacol. 1998;53:195–201.
Huang YH, Dykes-Hoberg M, Tanaka K, Rothstein JD, Bergles DE. Climbing fiber activation of EAAT4 transporters and kainate receptors in cerebellar Purkinje cells. J Neurosci. 2004;24:103–11.
Nikkuni O, Takayasu Y, Iino M, Tanaka K, Ozawa S. Facilitated activation of metabotropic glutamate receptors in cerebellar Purkinje cells in glutamate transporter EAAT4-deficient mice. Neurosci Res. 2007;59:296–303.
Hansel C, Linden DJ, D'Angelo E. Beyond parallel fiber LTD: the diversity of synaptic and non-synaptic plasticity in the cerebellum. Nat Neurosci. 2001;4:467–75.
Takayasu Y, Iino M, Kakegawa W, Maeno H, Watase K, Wada K, et al. Differential roles of glial and neuronal glutamate transporters in Purkinje cell synapses. J Neurosci. 2005;25:8788–93.
Watase K, Hashimoto K, Kano M, Yamada K, Watanabe M, Inoue Y, et al. Motor discoordination and increased susceptibility to cerebellar injury in GLAST mutant mice. Eur J Neurosci. 1998;10:976–88.
Tanaka K, Watase K, Manabe T, Yamada K, Watanabe M, Takahashi K, et al. Epilepsy and exacerbation of brain injury in mice lacking the glutamate transporter GLT-1. Science. 1997;276:1699–702.
Matsugami TR, Tanemura K, Mieda M, Nakatomi R, Yamada K, Kondo T, et al. From the Cover: Indispensability of the glutamate transporters GLAST and GLT1 to brain development. Proc Natl Acad Sci U S A. 2006;103:12161–6.
Wadiche JI, Amara SG, Kavanaugh MP. Ion fluxes associated with excitatory amino acid transport. Neuron. 1995;15:721–8.
Wadiche JI, Kavanaugh MP. Macroscopic and microscopic properties of a cloned glutamate transporter/chloride channel. J Neurosci. 1998;18:7650–61.
Auger C, Attwell D. Fast removal of synaptic glutamate by postsynaptic transporters. Neuron. 2000;28:547–58.
Acknowledgments
We thank Dr. Bo Xiao (Sichuang University, Chengdu, China) for providing Rheb1f/f mice and Dr. Xiaobing Yuan (Institute of Neuroscience, Chinese Academy of Sciences, Shanghai, China) for providing L7-Cre mice. We are grateful for helpful advice from members of Shen lab. We thank the Core Facilities of Zhejiang University Institute of Neuroscience for technical assistance. This work was supported by grants from the National Natural Science Foundation of China (31471024, 31271148, 81471398, and 31200818), Zhejiang Provincial Natural Science Foundation (LY15C090001), Seeds Fund for Interdisciplinary Research at Zhejiang University (JCZZ-2013037), Public Benefit Research Project of Science and Technology Department of Zhejiang Province (2013C33233), and Shenzhen Committee for Technological Renovation (ZDSY20120617112838879).
Conflict of Interest
All authors declare that there are no conflicts of interest on the paper.
Author information
Authors and Affiliations
Corresponding authors
Additional information
Nan-Wei Jiang, De-Juan Wang and Ya-Jun Xie contributed equally to this work.
Rights and permissions
About this article
Cite this article
Jiang, NW., Wang, DJ., Xie, YJ. et al. Downregulation of Glutamate Transporter EAAT4 by Conditional Knockout of Rheb1 in Cerebellar Purkinje Cells. Cerebellum 15, 314–321 (2016). https://doi.org/10.1007/s12311-015-0701-9
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12311-015-0701-9