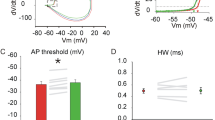
Abstract
Glutamate-receptor-like molecule delta2 (GluD2) is selectively expressed on the postsynaptic membranes at parallel fiber to Purkinje cell (PF-PC) synapses in the cerebellum. GluD2 plays critical roles not only in postsynaptic long-term depression but also in the induction of presynaptic differentiation through trans-synaptic interaction with neurexin. However, how GluD2 influences the presynaptic function remains unknown. Here, effects of the deletion of postsynaptic GluD2 on the presynaptic properties were studied focusing on the paired pulse ratio (PPR) of two consecutive EPSC amplitudes, which was larger in GluD2 knockout mice. The PPR difference remained even if saturation of glutamate binding to postsynaptic receptors was suppressed, confirming the presynaptic difference between the genotypes. We then explored the possibility that presynaptic voltage-gated Ca2+ channels (VGCCs) are affected in GluD2 knockout mice. Application of selective blockers for specific VGCCs indicated that R-type but not P/Q- or N-type VGCC, was affected in the mutant mice. Furthermore, presynaptic long-term potentiation (LTP) at PF-PC synapses, which requires R-type VGCC, was impaired in GluD2 knockout mice. These results suggest that GluD2 deletion impairs presynaptic R-type VGCC, resulting in decreased release of synaptic vesicles, and also in the impairment of presynaptic LTP at PF-PC synapses.






Similar content being viewed by others
References
Ito M. Cerebellar circuitry as a neuronal machine. Prog Neurobiol. 2006;78:272–303.
Takayama C, Nakagawa S, Watanabe M, Mishina M, Inoue Y. Light- and electron-microscopic localization of the glutamate receptor channel δ2 subunit in the mouse Purkinje cell. Neurosci Lett. 1995;188:89–92.
Araki K, Meguro H, Kushiya E, Takayama C, Inoue Y, Mishina M. Selective expression of the glutamate receptor channel δ2 subunit in cerebellar Purkinje cells. Biochem Biophys Res Commun. 1993;197:1267–76.
Lomeli H, Sprengel R, Laurie DJ, Köhr G, Herb A, Seeburg PH, et al. The rat delta-1 and delta-2 subunits extend the excitatory amino acid receptor family. FEBS Lett. 1993;315:318–22.
Schmid SM, Kott S, Sager C, Huelsken T, Hollmann M. The glutamate receptor subunit delta2 is capable of gating its intrinsic ion channel as revealed by ligand binding domain transplantation. Proc Natl Acad Sci U S A. 2009;106:10320–5.
Mandolesi G, Cesa R, Autuori E, Strata P. An orphan ionotropic glutamate receptor: the δ2 subunit. Neuroscience. 2009;158:67–77.
Yuzaki M. New (but old) molecules regulating synapse integrity and plasticity: Cbln1 and the δ2 glutamate receptor. Neuroscience. 2009;162:633–43.
Hirano T. Glutamate-receptor-like molecule GluRδ2 involved in synapse formation at parallel fiber-Purkinje neuron synapses. Cerebellum. 2012;11:71–7.
Kashiwabuchi N, Ikeda K, Araki K, Hirano T, Shibuki K, Takayama C, et al. Impairment of motor coordination, Purkinje cell synapse formation, and cerebellar long-term depression in GluRδ2 mutant mice. Cell. 1995;81:245–52.
Yawata S, Tsuchida H, Kengaku M, Hirano T. Membrane-proximal region of glutamate receptor δ2 subunit is critical for long-term depression and interaction with protein interacting with C kinase 1 in a cerebellar Purkinje neuron. J Neurosci. 2006;26:3626–33.
Kohda K, Kakegawa W, Matsuda S, Nakagami R, Kakiya N, Yuzaki M. The extreme C-terminus of GluRδ2 is essential for induction of long-term depression in cerebellar slices. Eur J Neurosci. 2007;25:1357–62.
Uemura T, Kakizawa S, Yamasaki M, Sakimura K, Watanabe M, Iino M, et al. Regulation of long-term depression and climbing fiber territory by glutamate receptor δ2 at parallel fiber synapses through its C-terminal domain in cerebellar Purkinje cells. J Neurosci. 2007;27:12096–108.
Torashima T, Iizuka A, Horiuchi H, Mitsumura K, Yamasaki M, Koyama C, et al. Rescue of abnormal phenotypes in δ2 glutamate receptor-deficient mice by the extracellular N-terminal and intracellular C-terminal domains of the δ2 glutamate receptor. Eur J Neurosci. 2009;30:355–65.
Kuroyanagi T, Yokoyama M, Hirano T. Postsynaptic glutamate receptor δ family contributes to presynaptic terminal differentiation and establishment of synaptic transmission. Proc Natl Acad Sci U S A. 2009;106:4912–6.
Uemura T, Lee SJ, Yasumura M, Takeuchi T, Yoshida T, Ra M, et al. Trans-synaptic interaction of GluRδ2 and Neurexin through Cbln1 mediates synapse formation in the cerebellum. Cell. 2010;141:1068–79.
Matsuda K, Miura E, Miyazaki T, Kakegawa W, Emi K, Narumi S, et al. Cbln1 is a ligand for an orphan glutamate receptor δ2, a bidirectional synapse organizer. Science. 2010;328:363–8.
Kuroyanagi T, Hirano T. Flap loop of GluD2 binds to Cbln1 and induces presynaptic differentiation. Biochem Biophys Res Commun. 2010;398:537–41.
Matsuda K, Yuzaki M. Cbln family proteins promote synapse formation by regulating distinct neurexin signaling pathways in various brain regions. Eur J Neurosci. 2011;33:1447–61.
Debanne D, Guérineau NC, Gähwiler BH, Thompson SM. Paired-pulse facilitation and depression at unitary synapses in rat hippocampus: quantal fluctuation affects subsequent release. J Physiol. 1996;491:163–76.
Hashimoto K, Kano M. Presynaptic origin of paired-pulse depression at climbing fibre-Purkinje cell synapses in the rat cerebellum. J Physiol. 1998;506:391–405.
Wadiche JI, Jahr CE. Multivesicular release at climbing fiber-Purkinje cell synapses. Neuron. 2001;32:301–13.
Foster KA, Crowley JJ, Regehr WG. The influence of multivesicular release and postsynaptic receptor saturation on transmission at granule cell to Purkinje cell synapses. J Neurosci. 2005;25:11655–65.
Missler M, Zhang W, Rohlmann A, Kattenstroth G, Hammer RE, Gottmann K, et al. α-neurexins couple Ca2+ channels to synaptic vesicle exocytosis. Nature. 2003;423:939–48.
Zhang W, Rohlmann A, Sargsyan V, Aramuni G, Hammer RE, Südhof TC, et al. Extracellular domains of α-neurexins participate in regulating synaptic transmission by selectively affecting N- and P/Q-type Ca2+ channels. J Neurosci. 2005;25:4330–42.
Mintz IM, Sabatini BL, Regehr WG. Calcium control of transmitter release at a cerebellar synapse. Neuron. 1995;15:675–88.
Myoga MH, Regehr WG. Calcium microdomains near R-type calcium channels control the induction of presynaptic long-term potentiation at parallel fiber to Purkinje cell synapses. J Neurosci. 2011;31:5235–43.
Coesmans M, Weber JT, De Zeeuw CI, Hansel C. Bidirectional parallel fiber plasticity in the cerebellum under climbing fiber control. Neuron. 2004;44:691–700.
Wall MJ, Dale N. Auto-inhibition of rat parallel fibre-Purkinje cell synapses by activity-dependent adenosine release. J Physiol. 2007;581:553–65.
Dittman JS, Regehr WG. Contributions of calcium-dependent and calcium-independent mechanisms to presynaptic inhibition at a cerebellar synapse. J Neurosci. 1996;16:1623–33.
Kreitzer AC, Regehr WG. Retrograde inhibition of presynaptic calcium influx by endogenous cannabinoids at excitatory synapses onto Purkinje cells. Neuron. 2001;29:717–27.
Hirano T. Differential pre- and postsynaptic mechanisms for synaptic potentiation and depression between a granule cell and a Purkinje cell in rat cerebellar culture. Synapse. 1991;7:321–3.
Qiu DL, Knöpfel T. An NMDA receptor/nitric oxide cascade in presynaptic parallel fiber-Purkinje neuron long-term potentiation. J Neurosci. 2007;27:3408–15.
Valera AM, Doussau F, Poulain B, Barbour B, Isope P. Adaptation of granule cell to Purkinje cell synapses to high-frequency transmission. J Neurosci. 2012;32:3267–80.
Yamasaki M, Miyazaki T, Azechi H, Abe M, Natsume R, Hagiwara T, et al. Glutamate receptor δ2 is essential for input pathway-dependent regulation of synaptic AMPAR contents in cerebellar Purkinje cells. J Neurosci. 2011;31:3362–74.
Augustine GJ. How does calcium trigger neurotransmitter release? Curr Opin Neurobiol. 2001;11:320–6.
Kochubey O, Lou X, Schneggenburger R. Regulation of transmitter release by Ca2+ and synaptotagmin: insights from a large CNS synapse. Trends Neurosci. 2011;34:237–46.
Brown SP, Safo PK, Regehr WG. Endocannabinoids inhibit transmission at granule cell to Purkinje cell synapses by modulating three types of presynaptic calcium channels. J Neurosci. 2004;24:5623–31.
Salin PA, Malenka RC, Nicoll RA. Cyclic AMP mediates a presynaptic form of LTP at cerebellar parallel fiber synapses. Neuron. 1996;16:797–803.
Chen C, Regehr WG. The mechanism of cAMP-mediated enhancement at a cerebellar synapse. J Neurosci. 1997;17:8687–94.
Hirano T, Kasono K, Araki K, Shinozuka K, Mishina M. Involvement of the glutamate receptor δ2 subunit in the long-term depression of glutamate responsiveness in cultured rat Purkinje cells. Neurosci Lett. 1994;182:172–6.
Boyden ES, Katoh A, Raymond JL. Cerebellum-dependent learning: the role of multiple plasticity mechanisms. Annu Rev Neurosci. 2004;27:581–609.
Ito M. Cerebellar long-term depression: characterization, signal transduction, and functional roles. Physiol Rev. 2001;81:1143–95.
Linden DJ. The expression of cerebellar LTD in culture is not associated with changes in AMPA-receptor kinetics, agonist affinity, or unitary conductance. Proc Natl Acad Sci U S A. 2001;98:14066–71.
Atluri PP, Regehr WG. Determinants of the time course of facilitation at the granule cell to Purkinje cell synapse. J Neurosci. 1996;16:5661–71.
Zucker RS, Regehr WG. Short-term synaptic plasticity. Annu Rev Physiol. 2002;64:355–405.
Catterall WA. Structure and regulation of voltage-gated Ca2+ channels. Annu Rev Cell Dev Biol. 2000;16:521–55.
Tedford HW, Zamponi GW. Direct G protein modulation of Cav2 calcium channels. Pharmacol Rev. 2006;58:837–62.
Li L, Bischofberger J, Jonas P. Differential gating and recruitment of P/Q-, N-, and R-type Ca2+ channels in hippocampal mossy fiber boutons. J Neurosci. 2007;27:13420–9.
Cao YQ, Piedras-Rentería ES, Smith GB, Chen G, Harata NC, Tsien RW. Presynaptic Ca2+ channels compete for channel type-preferring slots in altered neurotransmission arising from Ca2+ channelopathy. Neuron. 2004;43:387–400.
Cao YQ, Tsien RW. Different relationship of N- and P/Q-type Ca2+ channels to channel-interacting slots in controlling neurotransmission at cultured hippocampal synapses. J Neurosci. 2010;30:4536–46.
Miyawaki H, Hirano T. Different correlations among physiological and morphological properties at single glutamatergic synapses in the rat hippocampus and the cerebellum. Synapse. 2011;65:412–23.
Delaney AJ, Jahr CE. Kainate receptors differentially regulate release at two parallel fiber synapses. Neuron. 2002;36:475–82.
Bao J, Reim K, Sakaba T. Target-dependent feedforward inhibition mediated by short-term synaptic plasticity in the cerebellum. J Neurosci. 2010;30:8171–9.
Craig AM, Kang Y. Neurexin-neuroligin signaling in synapse development. Curr Opin Neurobiol. 2007;17:43–52.
Südhof TC. Neuroligins and neurexins link synaptic function to cognitive disease. Nature. 2008;455:903–11.
Linhoff MW, Laurén J, Cassidy RM, Dobie FA, Takahashi H, Nygaard HB, et al. An unbiased expression screen for synaptogenic proteins identifies the LRRTM protein family as synaptic organizers. Neuron. 2009;61:734–49.
Ko J, Soler-Llavina GJ, Fuccillo MV, Malenka RC, Südhof TC. Neuroligins/LRRTMs prevent activity- and Ca2+/calmodulin-dependent synapse elimination in cultured neurons. J Cell Biol. 2011;194:323–34.
Chubykin AA, Atasoy D, Etherton MR, Brose N, Kavalali ET, Gibson JR, et al. Activity-dependent validation of excitatory versus inhibitory synapses by neuroligin-1 versus neuroligin-2. Neuron. 2007;54:919–31.
Comoletti D, Flynn R, Jennings LL, Chubykin A, Matsumura T, Hasegawa H, et al. Characterization of the interaction of a recombinant soluble neuroligin-1 with neurexin-1β. J Biol Chem. 2003;278:50497–505.
Koehnke J, Jin X, Trbovic N, Katsamba PS, Brasch J, Ahlsen G, et al. Crystal structures of β-neurexin 1 and β-neurexin 2 ectodomains and dynamics of splice insertion sequence 4. Structure. 2008;16:410–21.
Acknowledgments
We thank Y. Tagawa, G. Ohtsuki, and E. Nakajima for comments on the manuscript. This work was supported by a grant-in-aid for scientific research and by a grant for excellent graduate schools from the Ministry of Education, Culture, Sports, Science and Technology in Japan, Takeda Science Foundation, and also by Global COE program A06 of Kyoto University.
Conflict of Interest
The authors declare no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Electronic Supplementary Material
Below is the link to the electronic supplementary material.
Supplemental Fig. 1
Effects of DGG on the EPSC amplitude. a, b The decrease in EPSC amplitude caused by application of 2 mM DGG in wild-type (“+/+” n = 5; a) and GluD2 knockout (“−/−” n = 5; b) mice (JPEG 9 kb)
Supplemental Fig. 2
The time courses of EPSC amplitude change when [Ca2+]o was increased in wild-type (“+/+” open circles, n = 5) and in knockout (“−/−” filled circles, n = 5) mice (JPEG 12 kb)
Supplemental Fig. 3
PPR with various intervals in the presence of both Ni2+ and DGG in wild-type (“+/+” open circles, n = 9) and in knockout (“−/−” filled circles, n = 9) mice (JPEG 7 kb)
Supplemental Fig. 4
Effects of a cocktail of antagonists for A1R, GABABR, and CB1R. Application of the cocktail increased the EPSC amplitude both in wild-type (“+/+” open circles, n = 5) and in knockout (“−/−” filled circles, n = 5) mice (JPEG 14 kb)
Rights and permissions
About this article
Cite this article
Yamashita, M., Kawaguchi, Sy. & Hirano, T. Contribution of Postsynaptic GluD2 to Presynaptic R-type Ca2+ Channel Function, Glutamate Release and Long-term Potentiation at Parallel Fiber to Purkinje Cell Synapses. Cerebellum 12, 657–666 (2013). https://doi.org/10.1007/s12311-013-0474-y
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12311-013-0474-y