Abstract
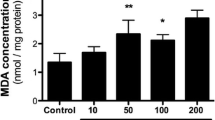
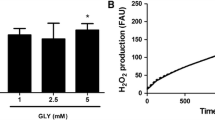
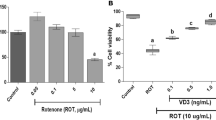
Phytanic acid (Phyt) brain concentrations are highly increased in Refsum disease, a peroxisomal disorder clinically characterized by neurological features, cardiac abnormalities, and retinitis pigmentosa. Considering that the pathogenesis of cerebellar ataxia, a common finding in this disease, is still unknown, in the present work we investigated the in vitro effects of Phyt at concentrations similar to those found in affected patients on important parameters of mitochondrial homeostasis in cerebellum from young rats. The respiratory parameters states 3 and 4 and respiratory control ratio (RCR) determined by oxygen consumption, membrane potential (∆Ψm), NAD(P)H pool content, and swelling were evaluated in mitochondrial preparations from this cerebral structure. Phyt markedly increased state 4 respiration, whereas state 3 respiration, the RCR, the mitochondrial matrix NAD(P)H content, and ∆Ψm were decreased by this fatty acid, being the latter effect partially prevented by N-acetylcysteine. These data indicate that Phyt behaves as an uncoupler of oxidative phosphorylation and as a metabolic inhibitor disrupting mitochondrial homeostasis in cerebellum. It is proposed that these pathomechanisms may contribute at least in part to the cerebellar alterations found in Refsum disease.





Similar content being viewed by others
Abbreviations
- ANT:
-
Adenine nucleotide translocator
- ATC:
-
Atractyloside
- NAC:
-
N-acetylcysteine
- Phyt:
-
Phytanic acid
References
Schönfeld P, Struy H. Refsum disease diagnostic marker phytanic acid alters the physical state of membrane proteins of liver mitochondria. FEBS Lett. 1999;457(2):179–83.
Wanders R, Jansen G, Skjeldal O. Refsum disease, peroxisomes and phytanic acid oxidation: a review. J Neuropathol Exp Neurol. 2001;60(11):1021–31.
Foulon V, Asselberghs S, Geens W, Mannaerts GP, Casteels M, Van Veldhoven PP. Further studies on the substrate spectrum of phytanoyl-CoA hydroxylase: implications for Refsum disease? J Lipid Res. 2003;44(12):2349–55.
Ferdinandusse S, Zomer AW, Komen JC, van den Brink CE, Thanos M, Hamers FP, et al. Ataxia with loss of Purkinje cells in a mouse model for Refsum disease. Proc Natl Acad Sci U S A. 2008;105(46):17712–7.
Wierzbicki A, Lloyd M, Schofield C, Feher M, Gibberd F. Refsum’s disease: a peroxisomal disorder affecting phytanic acid alpha-oxidation. J Neurochem. 2002;80(5):727–35.
Chow CW, Poulos A, Fellenberg AJ, Christodoulou J, Danks DM. Autopsy findings in two siblings with infantile Refsum disease. Acta Neuropathol. 1992;83(2):190–5.
Eldjarn L, Try K, Stokke O, Munthe-Kaas A, Refsum S, Steinberg D, et al. Dietary effects on serum-phytanic-acid levels and on clinical manifestations in heredopathia atactica polyneuritiformis. Lancet. 1966;1(7439):691–3.
Gibberd F, Billimoria J, Page N, Retsas S. Heredopathia atactica polyneuritiformis (Refsum’s disease) treated by diet and plasma-exchange. Lancet. 1979;1(8116):575–8.
Hungerbühler J, Meier C, Rousselle L, Quadri P, Bogousslavsky J. Refsum’s disease: management by diet and plasmapheresis. Eur Neurol. 1985;24(3):153–9.
Masters-Thomas A, Bailes J, Billimoria J, Clemens M, Gibberd F, Page N. Heredopathia atactica polyneuritiformis (Refsum’s disease): 1. Clinical features and dietary management. J Hum Nutr. 1980;34(4):245–50.
Komen J, Distelmaier F, Koopman W, Wanders R, Smeitink J, Willems P. Phytanic acid impairs mitochondrial respiration through protonophoric action. Cell Mol Life Sci. 2007;64(24):3271–81.
Kahlert S, Schönfeld P, Reiser G. The Refsum disease marker phytanic acid, a branched chain fatty acid, affects Ca2+ homeostasis and mitochondria, and reduces cell viability in rat hippocampal astrocytes. Neurobiol Dis. 2005;18(1):110–8.
Rönicke S, Kruska N, Kahlert S, Reiser G. The influence of the branched-chain fatty acids pristanic acid and Refsum disease-associated phytanic acid on mitochondrial functions and calcium regulation of hippocampal neurons, astrocytes, and oligodendrocytes. Neurobiol Dis. 2009;36(2):401–10.
Schönfeld P, Reiser G. Rotenone-like action of the branched-chain phytanic acid induces oxidative stress in mitochondria. J Biol Chem. 2006;281(11):7136–42.
Idel S, Ellinghaus P, Wolfrum C, Nofer J, Gloerich J, Assmann G, et al. Branched chain fatty acids induce nitric oxide-dependent apoptosis in vascular smooth muscle cells. J Biol Chem. 2002;277(51):49319–25.
Leipnitz G, Amaral A, Zanatta A, Seminotti B, Fernandes C, Knebel L, et al. Neurochemical evidence that phytanic acid induces oxidative damage and reduces the antioxidant defenses in cerebellum and cerebral cortex of rats. Life Sci. 2010;87(9–10):275–80.
Busanello E, Viegas C, Moura A, Tonin A, Grings M, Vargas C, et al. In vitro evidence that phytanic acid compromises Na(+), K(+)-ATPase activity and the electron flow through the respiratory chain in brain cortex from young rats. Brain Res. 2010;1352:231–8.
Rosenthal R, Hamud F, Fiskum G, Varghese P, Sharpe S. Cerebral ischemia and reperfusion: prevention of brain mitochondrial injury by lidoflazine. J Cereb Blood Flow Metab. 1987;7(6):752–8.
Amaral AU, Leipnitz G, Fernandes CG, Seminotti B, Schuck PF, Wajner M. Alpha-ketoisocaproic acid and leucine provoke mitochondrial bioenergetic dysfunction in rat brain. Brain Res. 2010;1324:75–84.
Schuck PF, Ferreira GC, Tonin AM, Viegas CM, Busanello EN, Moura AP, et al. Evidence that the major metabolites accumulating in medium-chain acyl-CoA dehydrogenase deficiency disturb mitochondrial energy homeostasis in rat brain. Brain Res. 2009;1296:117–26.
Maciel EN, Kowaltowski AJ, Schwalm FD, Rodrigues JM, Souza DO, Vercesi AE, et al. Mitochondrial permeability transition in neuronal damage promoted by Ca2+ and respiratory chain complex II inhibition. J Neurochem. 2004;90(5):1025–35.
Akerman KE, Wikström MK. Safranine as a probe of the mitochondrial membrane potential. FEBS Lett. 1976;68(2):191–7.
Kowaltowski AJ, Cosso RG, Campos CB, Fiskum G. Effect of Bcl-2 overexpression on mitochondrial structure and function. J Biol Chem. 2002;277(45):42802–7.
Schuck PF, Ferreira GC, Tahara EB, Klamt F, Kowaltowski AJ, Wajner M. cis-4-decenoic acid provokes mitochondrial bioenergetic dysfunction in rat brain. Life Sci. 2010;87(5–6):139–46.
Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–54.
Tran D, Greenhill W, Wilson S. Infantile refsum disease with enamel defects: a case report. Pediatr Dent. 2011;33(3):266–70.
Brustovetsky NN, Dedukhova VI, Egorova MV, Mokhova EN, Skulachev VP. Inhibitors of the ATP/ADP antiporter suppress stimulation of mitochondrial respiration and H+ permeability by palmitate and anionic detergents. FEBS Lett. 1990;272(1–2):187–9.
Khailova LS, Prikhodko EA, Dedukhova VI, Mokhova EN, Popov VN, Skulachev VP. Participation of ATP/ADP antiporter in oleate- and oleate hydroperoxide-induced uncoupling suppressed by GDP and carboxyatractylate. Biochim Biophys Acta. 2006;1757(9–10):1324–9.
Samartsev VN, Simonyan RA, Markova OV, Mokhova EN, Skulachev VP. Comparative study on uncoupling effects of laurate and lauryl sulfate on rat liver and skeletal muscle mitochondria. Biochim Biophys Acta. 2000;1459(1):179–90.
Skulachev VP. Uncoupling: new approaches to an old problem of bioenergetics. Biochim Biophys Acta. 1998;1363(2):100–24.
Skulachev VP. Fatty acid circuit as a physiological mechanism of uncoupling of oxidative phosphorylation. FEBS Lett. 1991;294(3):158–62.
Kadenbach B. Intrinsic and extrinsic uncoupling of oxidative phosphorylation. Biochim Biophys Acta. 2003;1604(2):77–94.
Schönfeld P, Kahlert S, Reiser G. In brain mitochondria the branched-chain fatty acid phytanic acid impairs energy transduction and sensitizes for permeability transition. Biochem J. 2004;383(Pt 1):121–8.
Ferdinandusse S, Rusch H, van Lint A, Dacremont G, Wanders R, Vreken P. Stereochemistry of the peroxisomal branched-chain fatty acid alpha- and beta-oxidation systems in patients suffering from different peroxisomal disorders. J Lipid Res. 2002;43(3):438–44.
Khan A, Wei X, Snyder F, Mah J, Waterham H, Wanders R. Neurodegeneration in d-bifunctional protein deficiency: diagnostic clues and natural history using serial magnetic resonance imaging. Neuroradiology. 2010;52(12):1163–6.
Verhoeven N, Jakobs C. Human metabolism of phytanic acid and pristanic acid. Prog Lipid Res. 2001;40(6):453–66.
Nicholls DG. Mitochondrial membrane potential and aging. Aging Cell. 2004;3(1):35–40.
Acknowledgments
We are grateful to the financial support of CNPq, CAPES, PROPESq/UFRGS, FAPERGS, PRONEX, FINEP Rede Instituto Brasileiro de Neurociência (IBN-Net) # 01.06.0842-00, and Instituto Nacional de Ciência e Tecnologia- Excitotoxicidade e Neuroproteção (INCT-EN).
Financial Support
CNPq, PROPESq/UFRGS, FAPERGS, PRONEX, FINEP Rede Instituto Brasileiro de Neurociência (IBN-Net) # 01.06.0842-00, and Instituto Nacional de Ciência e Tecnologia-Excitotoxicidade e Neuroproteção (INCT-EN).
Conflict of Interest Statement
The authors declare that there are no potencial conflicts of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Busanello, E.N.B., Amaral, A.U., Tonin, A.M. et al. Disruption of Mitochondrial Homeostasis by Phytanic Acid in Cerebellum of Young Rats. Cerebellum 12, 362–369 (2013). https://doi.org/10.1007/s12311-012-0426-y
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12311-012-0426-y