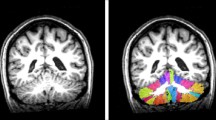
Abstract
Recent studies suggest that the role of the cerebellum extends into cognitive regulation and that subcortical vascular dementia (SVaD) can result in cerebellar atrophy. However, there has been no evaluation of the cerebellar volume in the preclinical stage of SVaD. We aimed to compare cerebellar volume among patients with amnestic mild cognitive impairment (aMCI) and subcortical vascular mild cognitive impairment (svMCI) and evaluate which factors could have contributed to the cerebellar volume. Participants were composed of 355 patients with aMCI, svMCI, Alzheimer's disease (AD), and SVaD. Cerebellar volumes were measured using automated methods. A direct comparison of the cerebellar volume in SVaD and AD groups showed that the SVaD group had a statistically smaller cerebellar volume than the AD group. Additionally, the svMCI group had a smaller cerebellar volume than the aMCI group, with the number of lacunes (especially in the supratentorial regions) being associated with cerebellar volume. Cerebellar volumes were associated with some neuropsychological tests, digit span backward and ideomotor apraxia. These findings suggest that cerebellar atrophy may be useful in differentiating subtypes of dementia and the cerebellum plays a potential role in cognition.


Similar content being viewed by others
References
Braak H, Braak E. Neuropathological stageing of Alzheimer-related changes. Acta Neuropathol. 1991;82:239–59.
Roman GC, Erkinjuntti T, Wallin A, Pantoni L, Chui HC. Subcortical ischaemic vascular dementia. Lancet Neurol. 2002;1:426–36.
Fazekas F, Chawluk JB, Alavi A, Hurtig HI, Zimmerman RA. MR signal abnormalities at 1.5 T in Alzheimer's dementia and normal aging. AJR Am J Roentgenol. 1987;149:351–6.
Dubois B, Albert ML. Amnestic MCI or prodromal Alzheimer's disease? Lancet Neurol. 2004;3:246–8.
Petersen RC, Smith GE, Waring SC, Ivnik RJ, Tangalos EG, Kokmen E. Mild cognitive impairment: clinical characterization and outcome. Arch Neurol. 1999;56:303–8.
Petersen RC, Doody R, Kurz A, Mohs RC, Morris JC, Rabins PV, et al. Current concepts in mild cognitive impairment. Arch Neurol. 2001;58:1985–92.
Frisoni GB, Galluzzi S, Bresciani L, Zanetti O, Geroldi C. Mild cognitive impairment with subcortical vascular features: clinical characteristics and outcome. J Neurol. 2002;249:1423–32.
Galluzzi S, Sheu CF, Zanetti O, Frisoni GB. Distinctive clinical features of mild cognitive impairment with subcortical cerebrovascular disease. Dement Geriatr Cogn Disord. 2005;19:196–203.
Debette S, Bombois S, Bruandet A, Delbeuck X, Lepoittevin S, Delmaire C, et al. Subcortical hyperintensities are associated with cognitive decline in patients with mild cognitive impairment. Stroke. 2007;38:2924–30.
Kim SH, Park JS, Ahn HJ, Seo SW, Lee JM, Kim ST, et al. Voxel-based analysis of diffusion tensor imaging in patients with subcortical vascular cognitive impairment: Correlates with cognitive and motor deficits. J Neuroimaging. 2010;21:317–24.
Baillieux H, De Smet HJ, Paquier PF, De Deyn PP, Marien P. Cerebellar neurocognition: insights into the bottom of the brain. Clin Neurol Neurosurg. 2008;110:763–73.
Kalashnikova LA, Zueva YV, Pugacheva OV, Korsakova NK. Cognitive impairments in cerebellar infarcts. Neurosci Behav Physiol. 2005;35:773–9.
Fox NC, Crum WR, Scahill RI, Stevens JM, Janssen JC, Rossor MN. Imaging of onset and progression of Alzheimer's disease with voxel-compression mapping of serial magnetic resonance images. Lancet. 2001;358:201–5.
McKhann G, Drachman D, Folstein M, Katzman R, Price D, Stadlan EM. Clinical diagnosis of Alzheimer's disease. Neurology. 1984;34:939.
Busser J, Geldmacher DS, Herrup K. Ectopic cell cycle proteins predict the sites of neuronal cell death in Alzheimer's disease brain. J Neurosci. 1998;18:2801.
Pantel J, Schroder J, Essig M, Jauss M, Schneider G, Eysenbach K, et al. In vivo quantification of brain volumes in subcortical vascular dementia and Alzheimer's disease. An MRI-based study. Dement Geriatr Cogn Disord. 1998;9:309–16.
Mielke R, Herholz K, Grond M, Kessler J, Heiss WD. Severity of vascular dementia is related to volume of metabolically impaired tissue. Arch Neurol. 1992;49:909–13.
Seo SW, Lee JM, Im K, Park JS, Kim SH, Kim ST, et al. Cortical thinning related to periventricular and deep white matter hyperintensities. Neurobiol Aging. 2012 (in press)
Du AT, Schuff N, Chao LL, Kornak J, Ezekiel F, Jagust WJ, et al. White matter lesions are associated with cortical atrophy more than entorhinal and hippocampal atrophy. Neurobiol Aging. 2005;26:553–9.
Seo SW, Cho SS, Park A, Chin J, Na DL. Subcortical vascular versus amnestic mild cognitive impairment: comparison of cerebral glucose metabolism. J Neuroimaging. 2009;19:213–9.
Seo SW, Im K, Lee JM, Kim YH, Kim ST, Kim SY, et al. Cortical thickness in single-versus multiple-domain amnestic mild cognitive impairment. NeuroImage. 2007;36:289–97.
Erkinjuntti T, Inzitari D, Pantoni L, Wallin A, Scheltens P, Rockwood K, et al. Research criteria for subcortical vascular dementia in clinical trials. J Neural Transm Suppl. 2000;59:23–30.
Sachdev P, Wen W, Shnier R, Brodaty H. Cerebral blood volume in T2-weighted white matter hyperintensities using exogenous contrast based perfusion MRI. J Neuropsychiatr Clin Neurosci. 2004;16:83.
Smith SM. Fast robust automated brain extraction. Hum Brain Mapp. 2002;17:143–55.
Collins DL, Neelin P, Peters TM, Evans AC. Automatic 3D intersubject registration of MR volumetric data in standardized Talairach space. J Comput Assist Tomogr. 1994;18:192–205.
Sled JG, Zijdenbos AP, Evans AC. A nonparametric method for automatic correction of intensity nonuniformity in MRI data. IEEE Trans Med Imaging. 1998;17:87–97.
Zijdenbos A, Forghani R, Evans A. Automatic quantification of MS lesions in 3D MRI brain data sets: validation of insect. MICCAI. 1998;98:439–48.
Tohka J, Zijdenbos A, Evans A. Fast and robust parameter estimation for statistical partial volume models in brain MRI. NeuroImage. 2004;23:84–97.
Yushkevich PA, Piven J, Hazlett HC, Smith RG, Ho S, Gee JC, et al. User-guided 3D active contour segmentation of anatomical structures: significantly improved efficiency and reliability. NeuroImage. 2006;31:1116–28.
Gonzalez RC, Woods RE, Eddins SL. Digital image processing using MATLAB. New Jersey: Pearson Prentice Hall; 2004.
Kang Y, Na D. Seoul neuropsychological screening battery. Incheon: Human Brain Research & Consulting Co.; 2003.
Schmahmann JD. The role of the cerebellum in cognition and emotion: personal reflections since 1982 on the dysmetria of thought hypothesis, and its historical evolution from theory to therapy. Neuropsychol Rev. 2010;20:236–60.
Heyder K, Suchan B, Daum I. Cortico-subcortical contributions to executive control. Acta Psychol. 2004;115:271–89.
Jouvent E, Viswanathan A, Mangin JF, O'Sullivan M, Guichard JP, Gschwendtner A, et al. Brain atrophy is related to lacunar lesions and tissue microstructural changes in CADASIL. Stroke. 2007;38:1786–90.
Heyder K, Suchan B, Daum I. Cortico-subcortical contributions to executive control. Acta Psychol (Amst). 2004;115:271–89.
Schmahmann JD, Pandya DN. The cerebrocerebellar system. Int Rev Neurobiol. 1997;41:31–60.
Infeld B, Davis SM, Lichtenstein M, Mitchell PJ, Hopper JL. Crossed cerebellar diaschisis and brain recovery after stroke. Stroke. 1995;26:90–5.
Tien R, Ashdown B. Crossed cerebellar diaschisis and crossed cerebellar atrophy: correlation of MR findings, clinical symptoms, and supratentorial diseases in 26 patients. Am J Roentgenol. 1992;158:1155–9.
Lin DD, Kleinman JT, Wityk RJ, Gottesman RF, Hillis AE, Lee AW, et al. Crossed cerebellar diaschisis in acute stroke detected by dynamic susceptibility contrast MR perfusion imaging. AJNR Am J Neuroradiol. 2009;30:710–5.
Tatsch K, Koch W, Linke R, Poepperl G, Peters N, Holtmannspoetter M, et al. Cortical hypometabolism and crossed cerebellar diaschisis suggest subcortically induced disconnection in CADASIL: an 18F-FDG PET study. J Nucl Med. 2003;44:862–9.
Ravizza SM, McCormick CA, Schlerf JE, Justus T, Ivry RB, Fiez JA. Cerebellar damage produces selective deficits in verbal working memory. Brain. 2006;129:306.
Choi S, Na DL, Kang E, Lee K, Lee S, Na D. Functional magnetic resonance imaging during pantomiming tool-use gestures. Exp Brain Res. 2001;139:311–7.
Acknowledgments
This study was supported by a grant from the Korean Healthcare Technology R&D Project; Ministry for Health, Welfare and Family Affairs, Republic of Korea (A102065 & A070001); a Korean Science and Engineering Foundation (KOSEF) NRL program grant funded by the Korean government (MEST; R0A-2007-000-20068-0); and a Samsung Medical Center Clinical Research Development Program grant (CRL-108011 & CRS 110-14-1).
Disclosure
The authors declare no conflicts of interest, and all protocols described in this study were approved by the Institutional Review Board of the Samsung Medical Center.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Yoon, C.W., Seo, S.W., Park, JS. et al. Cerebellar Atrophy in Patients with Subcortical-Type Vascular Cognitive Impairment. Cerebellum 12, 35–42 (2013). https://doi.org/10.1007/s12311-012-0388-0
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12311-012-0388-0