Abstract
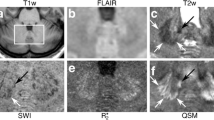
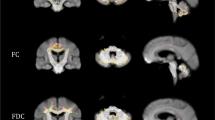
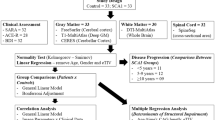
Magnetic resonance (MR) imaging is widely used to visualize atrophic processes that occur during the pathogenesis of spinocerebellar ataxias (SCAs). T1-weighted images are utilized to rate the atrophy of cerebellar vermis, cerebellar hemispheres, pons and midbrain. Signal changes in the basal ganglia and ponto-cerebellar fibers are evaluated by T2-weighted and proton density-weighted images. However, two-dimensional (2D) images do not allow a reliable quantification of the degree of atrophy. The latter is now possible through the application of three-dimensional (3D) true volumetric methods, which should be used for research purposes. Ideally, these methods should allow automated segmentation of contrast-defined boundaries by using region growing algorithms, which can be applied successfully in structures of the posterior fossa and basal ganglia. Thin slice thickness helps to minimize partial volume effects. Whereas volumetric approaches rely on predetermined anatomical boundaries, voxel-based morphometry has been developed to determine group differences between different types of SCA (cross-sectional studies) or within one SCA entity (longitudinal studies). We will review recent results and how these methods are currently used to (i) separate sporadic and dominantly inherited forms of cerebellar ataxias; (ii) identify specific SCA genotypes; (iii) correlate patho-anatomical changes with SCA disease symptoms or severity; and (iv) visualize and estimate the rate of progression in SCA.
Similar content being viewed by others
References
Schulz JB, Klockgether T, Petersen D, Jauch M, Müller-Schauenburg W, Spieker S, Voigt K, Dichgans J. Multiple system atrophy: Natural history, MRI morphology, and dopamine receptor imaging with 123IBZM-SPECT. J Neurol Neurosurg Psychiatry. 1994;57:1047–56.
Schrag A, Good CD, Miszkiel K, Morris HR, Mathias CJ, Lees AJ, Quinn NP. Differentiation of atypical parkinsonian syndromes with routine MRI. Neurology. 2000;54:697–702.
Payami H, Nutt J, Gancher S, Bird T, McNeal MG, Seltzer WK, Hussey J, Lockhart P, Gwinn-Hardy K, Singleton AA, Singleton AB, Hardy J, Farrer M. SCA2 may present as levodopa-responsive parkinsonism. Mov Disord. 2003;18:425–9.
Loy CT, Sweeney MG, Davis MB, Wills AJ, Sawle GV, Lees AJ, Tabrizi SJ. Spinocerebellar ataxia type 17: Extension of phenotype with putaminal rim hyperintensity on magnetic resonance imaging. Mov Disord. 2005;20:1521–3.
Imon Y, Katayama S, Kawakami H, Murata Y, Oka M, Nakamura S. A necropsied case of Machado-Joseph disease with a hyperintense signal of transverse pontine fibres on long TR sequences of magnetic resonance images. J Neurol Neurosurg Psychiatry. 1998;64:140–1.
Burk K, Skalej M, Dichgans J. Pontine MRI hyperintensities (‘the cross sign’) are not pathognomonic for multiple system atrophy (MSA). Mov Disord. 2001;16:535.
Murata Y, Yamaguchi S, Kawakami H, Imon Y, Maruyama H, Sakai T, Kazuta T, Ohtake T, Nishimura M, Saida T, Chiba S, Oh-i T, Nakamura S. Characteristic magnetic resonance imaging findings in Machado-Joseph disease. Arch Neurol. 1998;55:33–7.
Luft AR, Skalej M, Welte D, Kolb R, Bürk K, Schulz JB, Klockgether T, Voigt K. A semi-automated, three dimensional technique allowing quantification of cerebellar volume and its substructure using MRI. Magn Reson Med. 1998;40:143–51.
Luft AR, Skalej M, Welte D, Kolb R. Reliability and exactness of MRI-based volumetry: A phantom study. J Magn Reson Imag. 1996;6:700–04.
Klockgether T, Skalej M, Wedekind D, Luft A, Welte D, Schulz J, Abele M, Bürk K, Laccone F, Brice A, Dichgans J. Autosomal dominant cerebellar ataxia type I: MRI-based volumetry of posterior fossa structures and basal ganglia in SCA1, SCA2, and SCA3. Brain. 1998;121:1687–93.
Schulz JB, Skalej M, Wedekind D, Luft AR, Abele M, Voigt K, Dichgans J, Klockgether T. MRI-based volumetry differentiates idiopathic Parkinson’s syndrome from MSA and PSP. Ann Neurol. 1999;45:65–74.
Hauser TK, Luft A, Skalej M, Nagele T, Kircher TT, Leube DT, Schulz JB. Visualization and quantification of disease progression in multiple system atrophy. Mov Disord. 2006;21:1674–81.
Seppi K, Schocke MF, Esterhammer R, Kremser C, Brenneis C, Mueller J, Boesch S, Jaschke W, Poewe W, Wenning GK. Diffusion-weighted imaging discriminates progressive supranuclear palsy from PD, but not from the parkinson variant of multiple system atrophy. Neurology. 2003;60:922–7.
Schocke MF, Seppi K, Esterhammer R, Kremser C, Jaschke W, Poewe W, Wenning GK. Diffusion-weighted MRI differentiates the Parkinson variant of multiple system atrophy from PD. Neurology. 2002;58:575–80.
Paviour DC, Thornton JS, Lees AJ, Jager HR. Diffusionweighted magnetic resonance imaging differentiates Parkinsonian variant of multiple-system atrophy from progressive supranuclear palsy. Mov Disord. 2007;22:68–74.
Nicoletti G, Lodi R, Condino F, Tonon C, Fera F, Malucelli E, Manners D, Zappia M, Morgante L, Barone P, Barbiroli B, Quattrone A. Apparent diffusion coefficient measurements of the middle cerebellar peduncle differentiate the Parkinson variant of MSA from Parkinson’s disease and progressive supranuclear palsy. Brain. 2006;129:2679–87.
Guerrini L, Lolli F, Ginestroni A, Belli G, Della Nave R, Tessa C, Foresti S, Cosottini M, Piacentini S, Salvi F, Plasmati R, De Grandis D, Siciliano G, Filla A, Mascalchi M. Brainstem neurodegeneration correlates with clinical dysfunction in SCA1 but not in SCA2. A quantitative volumetric, diffusion and proton spectroscopy MR study. Brain. 2004;127:1785–95.
Brenneis C, Bosch SM, Schocke M, Wenning GK, Poewe W. Atrophy pattern in SCA2 determined by voxel-based morphometry. Neuroreport. 2003;14:1799–802.
Lukas C, Schols L, Bellenberg B, Rub U, Przuntek H, Schmid G, Koster O, Suchan B. Dissociation of grey and white matter reduction in spinocerebellar ataxia type 3 and 6: A voxel-based morphometry study. Neurosci Lett. 2006;408:230–5.
Lasek K, Lencer R, Gaser C, Hagenah J, Walter U, Wolters A, Kock N, Steinlechner S, Nagel M, Zuhlke C, Nitschke MF, Brockmann K, Klein C, Rolfs A, Binkofski F. Morphological basis for the spectrum of clinical deficits in spinocerebellar ataxia 17 (SCA17). Brain. 2006;129:2341–52.
Schols L, Bauer P, Schmidt T, Schulte T, Riess O. Autosomal dominant cerebellar ataxias: clinical features, genetics, and pathogenesis. Lancet Neurol. 2004;3:291–304.
Burk K, Abele M, Fetter M, Dichgans J, Skalej M, Laccone F, Didierjean O, Brice A, Klockgether T. Autosomal dominant cerebellar ataxia type I clinical features and MRI in families with SCA1, SCA2 and SCA3. Brain. 1996;119 (Pt 5):1497–505.
Adachi M, Kawanami T, Ohshima H, Hosoya T. Characteristic signal changes in the pontine base on T2- and multishot diffusion-weighted images in spinocerebellar ataxia type 1. Neuroradiology. 2006;48:8–13.
Shan DE, Soong BW, Sun CM, Lee SJ, Liao KK, Liu RS. Spinocerebellar ataxia type 2 presenting as familial levodoparesponsive parkinsonism. Ann Neurol. 2001;50:812–15.
Ying SH, Choi SI, Perlman SL, Baloh RW, Zee DS, Toga AW. Pontine and cerebellar atrophy correlate with clinical disability in SCA2. Neurology. 2006;66:424–6.
Giuffrida S, Saponara R, Restivo DA, Trovato Salinaro A, Tomarchio L, Pugliares P, Fabbri G, Maccagnano C. Supratentorial atrophy in spinocerebellar ataxia type 2: MRI study of 20 patients. J Neurol. 1999;246:383–8.
Hellenbroich Y, Bubel S, Pawlack H, Opitz S, Vieregge P, Schwinger E, Zuhlke C. Refinement of the spinocerebellar ataxia type 4 locus in a large German family and exclusion of CAG repeat expansions in this region. J Neurol. 2003;250:668–71.
Hellenbroich Y, Gierga K, Reusche E, Schwinger E, Deller T, de Vos RA, Zuhlke C, Rub U. Spinocerebellar ataxia type 4 (SCA4): Initial pathoanatomical study reveals widespread cerebellar and brainstem degeneration. J Neural Transmiss (Vienna, Austria). 2006;113:829–43.
Burk K, Zuhlke C, Konig IR, Ziegler A, Schwinger E, Globas C, Dichgans J, Hellenbroich Y. Spinocerebellar ataxia type 5: Clinical and molecular genetic features of a German kindred. Neurology. 2004;62:327–9.
Ikeda Y, Dick KA, Weatherspoon MR, Gincel D, Armbrust KR, Dalton JC, Stevanin G, Durr A, Zuhlke C, Burk K, Clark HB, Brice A, Rothstein JD, Schut LJ, Day JW, Ranum LP. Spectrin mutations cause spinocerebellar ataxia type 5. Nat Genet. 2006;38:184–90.
Stevanin G, Herman A, Brice A, Durr A. Clinical and MRI findings in spinocerebellar ataxia type 5. Neurology. 1999;53:1355–7.
Schols L, Kruger R, Amoiridis G, Przuntek H, Epplen JT, Riess O. Spinocerebellar ataxia type 6: genotype and phenotype in German kindreds. J Neurol Neurosurg Psychiatry. 1998;64:67–73.
Satoh JI, Tokumoto H, Yukitake M, Matsui M, Matsuyama Z, Kawakami H, Nakamura S, Kuroda Y. Spinocerebellar ataxia type 6: MRI of three Japanese patients. Neuroradiology. 1998;40:222–7.
Butteriss D, Chinnery P, Birchall D. Radiological characterization of spinocerebellar ataxia type 6. Br J Radiol. 2005;78:694–6.
Murata Y, Kawakami H, Yamaguchi S, Nishimura M, Kohriyama T, Ishizaki F, Matsuyama Z, Mimori Y, Nakamura S. Characteristic magnetic resonance imaging findings in spinocerebellar ataxia 6. Arch Neurol. 1998;55:1348–52.
Nakagawa N, Katayama T, Makita Y, Kuroda K, Aizawa H, Kikuchi K. A case of spinocerebellar ataxia type 6 mimicking olivopontocerebellar atrophy. Neuroradiology. 1999;41:501–03.
Bang OY, Lee PH, Kim SY, Kim HJ, Huh K. Pontine atrophy precedes cerebellar degeneration in spinocerebellar ataxia 7: MRI-based volumetric analysis. J Neurol Neurosurg Psychiatry. 2004;75:1452–6.
Schols L, Bauer I, Zuhlke C, Schulte T, Kolmel C, Burk K, Topka H, Bauer P, Przuntek H, Riess O. Do CTG expansions at the SCA8 locus cause ataxia? Ann Neurol. 2003;54:110–15.
Ikeda Y, Shizuka M, Watanabe M, Okamoto K, Shoji M. Molecular and clinical analyses of spinocerebellar ataxia type 8 in Japan. Neurology. 2000;54:950–5.
Rasmussen A, Matsuura T, Ruano L, Yescas P, Ochoa A, Ashizawa T, Alonso E. Clinical and genetic analysis of four Mexican families with spinocerebellar ataxia type 10. Ann Neurol. 2001;50:234–9.
Worth PF, Giunti P, Gardner-Thorpe C, Dixon PH, Davis MB, Wood NW. Autosomal dominant cerebellar ataxia type III: linkage in a large British family to a 7.6-cM region on chromosome 15q14-21.3. Am J Hum Gen. 1999;65:420–6.
O’Hearn E, Holmes SE, Calvert PC, Ross CA, Margolis RL. SCA-12: Tremor with cerebellar and cortical atrophy is associated with a CAG repeat expansion. Neurology. 2001;56:299–303.
Stevanin G, Durr A, Benammar N, Brice A. Spinocerebellar ataxia with mental retardation (SCA13). Cerebellum. 2005;4:43–6.
Klebe S, Durr A, Rentschler A, Hahn-Barma V, Abele M, Bouslam N, Schols L, Jedynak P, Forlani S, Denis E, Dussert C, Agid Y, Bauer P, Globas C, Wullner U, Brice A, Riess O, Stevanin G. New mutations in protein kinase Cgamma associated with spinocerebellar ataxia type 14. Ann Neurol. 2005;58:720–9.
van de Warrenburg BP, Verbeek DS, Piersma SJ, Hennekam FA, Pearson PL, Knoers NV, Kremer HP, Sinke RJ. Identification of a novel SCA14 mutation in a Dutch autosomal dominant cerebellar ataxia family. Neurology. 2003;61:1760–5.
Knight MA, Gardner RJ, Bahlo M, Matsuura T, Dixon JA, Forrest SM, Storey E. Dominantly inherited ataxia and dysphonia with dentate calcification: Spinocerebellar ataxia type 20. Brain. 2004;127:1172–81.
Miyoshi Y, Yamada T, Tanimura M, Taniwaki T, Arakawa K, Ohyagi Y, Furuya H, Yamamoto K, Sakai K, Sasazuki T, Kira J. A novel autosomal dominant spinocerebellar ataxia (SCA16) linked to chromosome 8q22.1-24.1. Neurology. 2001;57:96–100.
Toyoshima Y, Yamada M, Onodera O, Shimohata M, Inenaga C, Fujita N, Morita M, Tsuji S, Takahashi H. SCA17 homozygote showing Huntington’s disease-like phenotype. Ann Neurol. 2004;55:281–6.
Rolfs A, Koeppen AH, Bauer I, Bauer P, Buhlmann S, Topka H, Schols L, Riess O. Clinical features and neuropathology of autosomal dominant spinocerebellar ataxia (SCA17). Ann Neurol. 2003;54:367–75.
Minnerop M, Joe A, Lutz M, Bauer P, Urbach H, Helmstaedter C, Reinhardt M, Klockgether T, Wullner U. Putamen dopamine transporter and glucose metabolism are reduced in SCA17. Ann Neurol. 2005;58:490–1.
Gunther P, Storch A, Schwarz J, Sabri O, Steinbach P, Wagner A, Hesse S. Basal ganglia involvement of a patient with SCA 17 – a new form of autosomal dominant spinocerebellar ataxia. J Neurol. 2004;251:896–7.
Brkanac Z, Fernandez M, Matsushita M, Lipe H, Wolff J, Bird TD, Raskind WH. Autosomal dominant sensory/motor neuropathy with Ataxia (SMNA): Linkage to chromosome 7q22-q32. Am J Med Genetics. 2002;114:450–7.
Schelhaas HJ, Verbeek DS, Van de Warrenburg BP, Sinke RJ. SCA19 and SCA22: Evidence for one locus with a worldwide distribution. Brain. 2004;127:E6; author reply E7.
Schelhaas HJ, van de Warrenburg BP. Clinical, psychological, and genetic characteristics of spinocerebellar ataxia type 19 (SCA19). Cerebellum. 2005;4:51–4.
Chung MY, Lu YC, Cheng NC, Soong BW. A novel autosomal dominant spinocerebellar ataxia (SCA22) linked to chromosome 1p21-q23. Brain. 2003;126:1293–9.
Devos D, Schraen-Maschke S, Vuillaume I, Dujardin K, Naze P, Willoteaux C, Destee A, Sablonniere B. Clinical features and genetic analysis of a new form of spinocerebellar ataxia. Neurology. 2001;56:234–8.
Verbeek DS, van de Warrenburg BP, Wesseling P, Pearson PL, Kremer HP, Sinke RJ. Mapping of the SCA23 locus involved in autosomal dominant cerebellar ataxia to chromosome region 20p13-12.3. Brain. 2004;127:2551–7.
Stevanin G, Bouslam N, Thobois S, Azzedine H, Ravaux L, Boland A, Schalling M, Broussolle E, Durr A, Brice A. Spinocerebellar ataxia with sensory neuropathy (SCA25) maps to chromosome 2p. Ann Neurol. 2004;55:97–104.
Yu GY, Howell MJ, Roller MJ, Xie TD, Gomez CM. Spinocerebellar ataxia type 26 maps to chromosome 19p13.3 adjacent to SCA6. Ann Neurol. 2005;57:349–54.
van Swieten JC, Brusse E, de Graaf BM, Krieger E, van de Graaf R, de Koning I, Maat-Kievit A, Leegwater P, Dooijes D, Oostra BA, Heutink P. A mutation in the fibroblast growth factor 14 gene is associated with autosomal dominant cerebellar ataxia (corrected). Am J Hum Genet. 2003;72:191–9.
Cagnoli C, Mariotti C, Taroni F, Seri M, Brussino A, Michielotto C, Grisoli M, Di Bella D, Migone N, Gellera C, Di Donato S, Brusco A. SCA28, a novel form of autosomal dominant cerebellar ataxia on chromosome 18p11.22-q11.2. Brain. 2006;129:235–42.
Onodera O, Idezuka J, Igarashi S, Takiyama Y, Endo K, Takano H, Oyake M, Tanaka H, Inuzuka T, Hayashi T, Yuasa T, Ito J, Miyatake T, Tsuji S. Progressive atrophy of cerebellum and brainstem as a function of age and the size of the expanded CAG repeats in the MJD1 gene in Machado-Joseph disease. Ann Neurol. 1998;43:288–96.
Schmitz-Hubsch T, du Montcel ST, Baliko L, Berciano J, Boesch S, Depondt C, Giunti P, Globas C, Infante J, Kang JS, Kremer B, Mariotti C, Melegh B, Pandolfo M, Rakowicz M, Ribai P, Rola R, Schols L, Szymanski S, van de Warrenburg BP, Durr A, Klockgether T, Fancellu R. Scale for the assessment and rating of ataxia: Development of a new clinical scale. Neurology. 2006;66:1717–20.
Schmitz-Hubsch T, Tezenas du Montcel S, Baliko L, Boesch S, Bonato S, Fancellu R, Giunti P, Globas C, Kang JS, Kremer B, Mariotti C, Melegh B, Rakowicz M, Rola R, Romano S, Schols L, Szymanski S, van de Warrenburg BP, Zdzienicka E, Durr A, Klockgether T. Reliability and validity of the International Cooperative Ataxia Rating Scale: A study in 156 spinocerebellar ataxia patients. Mov Disord. 2006;21:699–704.
Gröschel K, Kastrup A, Litvan I, Schulz JB. Penguins and hummingbirds: Midbrain atrophy in progressive supranuclear palsy. Neurology. 2006;66:949–50.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Döhlinger, S., Hauser, TK., Borkert, J. et al. Magnetic resonance imaging in spinocerebellar ataxias. Cerebellum 7, 204–214 (2008). https://doi.org/10.1007/s12311-008-0025-0
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12311-008-0025-0