Abstract
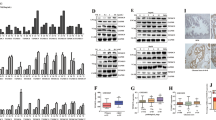
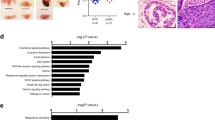
Reactive oxygen species (ROS) are implicated in many human diseases, including cancer. We have previously demonstrated that ROS increased the expression and activity of the chemokine receptor, CXCR4, which enhanced metastatic functions in prostate cancer cells. Studies have also revealed that CXCR4 and its ligand, SDF-1α, promoted ROS accumulation; however the source of ROS was not investigated. Recent evidence suggested that ROS accumulation in prostate cancer cell lines was contributed by the NADPH oxidase (NOX) family of enzymes. Herein, we sought to determine whether the CXCR4/SDF-1α signaling axis mediates ROS production through NOX in prostate cancer. We observed an increase in intracellular ROS generation in prostate cancer cells upon SDF-1α stimulation compared to untreated samples. Conversely, lower levels of ROS were detected in cells treated with AMD3100 (CXCR4 antagonist) or the ROS scavenger, N-acetyl-cysteine (NAC). Markedly reduced levels of ROS were observed in cells treated with apocynin (NOX inhibitor) compared to rotenone (mitochondrial complex I inhibitor)-treated cells. Specifically, we determined that NOX2 responded to, and was regulated by, the SDF-1α/CXCR4 signaling axis. Moreover, chemical inhibition of the ERK1/2 and PI3K pathways revealed that PI3K/AKT signaling participated in CXCR4-mediated NOX activity, and that these collective signaling events resulted in enhanced cell movement towards a chemoattractant. Finally, NOX2 may be a potential therapeutic target, as Oncomine microarray database analysis of normal prostate, benign prostatic hyperplasia (BPH) and prostatic intraepithelial neoplasia (PIN) tissue samples determined a correlation between NOX2 expression and prostate cancer. Taken together, these results suggest that CXCR4/SDF-1α-mediated ROS production through NOX2 enzymes may be an emerging concept by which chemokine signaling progresses tumorigenesis.








Similar content being viewed by others
References
Bedard K, Krause KH (2007) The NOX family of ROS-generating NADPH oxidases: physiology and pathophysiology. Physiol rev 87(1):245–313. doi:10.1152/physrev.00044.2005
Hordijk PL (2006) Regulation of NADPH oxidases. Circ Res 98(4):453–462. doi:10.1161/01.RES.0000204727.46710.5e
Ray PD, Huang BW, Tsuji Y (2012) Reactive oxygen species (ROS) homeostasis and redox regulation in cellular signaling. Cell signal 24(5):981–990. doi:10.1016/j.cellsig.2012.01.008
Yang Y, Karakhanova S, Werner J, Bazhin AV (2013) Reactive oxygen species in cancer biology and anticancer therapy. Curr med chem
Chetram MA, Don-Salu-Hewage AS, Hinton CV (2011) ROS enhances CXCR4-mediated functions through inactivation of PTEN in prostate cancer cells. Biochem biophys res commun 410(2):195–200. doi:10.1016/j.bbrc.2011.05.074
Teicher BA, Fricker SP (2010) CXCL12 (SDF-1)/CXCR4 pathway in cancer. Clin cancer res off j Am Assoc Cancer Res 16(11):2927–2931. doi:10.1158/1078-0432.CCR-09-2329
Kumar B, Koul S, Khandrika L, Meacham RB, Koul HK (2008) Oxidative stress is inherent in prostate cancer cells and is required for aggressive phenotype. Cancer Res 68(6):1777–1785. doi:10.1158/0008-5472.can-07-5259
Muller A, Homey B, Soto H, Ge N, Catron D, Buchanan ME, McClanahan T, Murphy E, Yuan W, Wagner SN, Barrera JL, Mohar A, Verastegui E, Zlotnik A (2001) Involvement of chemokine receptors in breast cancer metastasis. Nature 410(6824):50–56. doi:10.1038/35065016
Busillo JM, Benovic JL (2007) Regulation of CXCR4 signaling. Biochim Biophys Acta (BBA) Biomembr 1768(4):952–963. doi:10.1016/j.bbamem.2006.11.002
Sutton A, Friand V, Brulé-Donneger S, Chaigneau T, Ziol M, Sainte-Catherine O, Poiré A, Saffar L, Kraemer M, Vassy J, Nahon P, Salzmann J-L, Gattegno L, Charnaux N (2007) Stromal cell–derived factor-1/chemokine (C-X-C motif) ligand 12 stimulates human hepatoma cell growth, migration, and invasion. Mol Cancer Res 5(1):21–33. doi:10.1158/1541-7786.mcr-06-0103
Block K, Gorin Y (2012) Aiding and abetting roles of NOX oxidases in cellular transformation. Nat Rev Cancer 12(9):627–637
Takac I, Schroder K, Brandes RP (2012) The Nox family of NADPH oxidases: friend or foe of the vascular system? Curr hypertens rep 14(1):70–78. doi:10.1007/s11906-011-0238-3
Juhasz A, Ge Y, Markel S, Chiu A, Matsumoto L, van Balgooy J, Roy K, Doroshow JH (2009) Expression of NADPH oxidase homologues and accessory genes in human cancer cell lines, tumours and adjacent normal tissues. Free radic res 43(6):523–532. doi:10.1080/10715760902918683
Urao N, Inomata H, Razvi M, Kim HW, Wary K, McKinney R, Fukai T, Ushio-Fukai M (2008) Role of Nox2-based NADPH oxidase in bone marrow and progenitor cell function involved in neovascularization induced by hindlimb ischemia. Circ Res 103(2):212–220. doi:10.1161/circresaha.108.176230
Wu HC, Hsieh JT, Gleave ME, Brown NM, Pathak S, Chung LW (1994) Derivation of androgen-independent human LNCaP prostatic cancer cell sublines: role of bone stromal cells. Int j cancer 57(3):406–412
Chetram MA, Odero-Marah V, Hinton CV (2011) Loss of PTEN permits CXCR4-mediated tumorigenesis through ERK1/2 in prostate cancer cells. Mol cancer res: MCR 9(1):90–102. doi:10.1158/1541-7786.MCR-10-0235
Ke Q, Costa M (2006) Hypoxia-inducible factor-1 (HIF-1). Mol Pharmacol 70(5):1469–1480. doi:10.1124/mol.106.027029
Lee SR, Yang KS, Kwon J, Lee C, Jeong W, Rhee SG (2002) Reversible inactivation of the tumor suppressor PTEN by H2O2. J biol chem 277(23):20336–20342. doi:10.1074/jbc.M111899200
Silva A, Yunes JA, Cardoso BA, Martins LR, Jotta PY, Abecasis M, Nowill AE, Leslie NR, Cardoso AA, Barata JT (2008) PTEN posttranslational inactivation and hyperactivation of the PI3K/Akt pathway sustain primary T cell leukemia viability. J clin investig 118(11):3762–3774. doi:10.1172/JCI34616
Chetram MA, Hinton CV (2012) ROS-mediated regulation of CXCR4 in cancer. Front Biol
Lee RL, Westendorf J, Gold MR (2007) Differential role of reactive oxygen species in the activation of mitogen-activated protein kinases and Akt by key receptors on B-lymphocytes: CD40, the B cell antigen receptor, and CXCR4. J Cell Commun Signal 1(1):33–43. doi:10.1007/s12079-007-0006-y
Lin W, Wu G, Li S, Weinberg EM, Kumthip K, Peng LF, Méndez-Navarro J, Chen WC, Jilg N, Zhao H, Goto K, Zhang L, Brockman MA, Schuppan D, Chung RT (2011) HIV and HCV cooperatively promote hepatic fibrogenesis via induction of reactive oxygen species and NFkappaB. J biol chem 286(4):2665–2674. doi:10.1074/jbc.M110.168286
Dar A, Schajnovitz A, Lapid K, Kalinkovich A, Itkin T, Ludin A, Kao WM, Battista M, Tesio M, Kollet O, Cohen NN, Margalit R, Buss EC, Baleux F, Oishi S, Fujii N, Larochelle A, Dunbar CE, Broxmeyer HE, Frenette PS, Lapidot T (2011) Rapid mobilization of hematopoietic progenitors by AMD3100 and catecholamines is mediated by CXCR4-dependent SDF-1 release from bone marrow stromal cells. Leukemia. doi:10.1038/leu.2011.62
Chetram MA, Don-Salu-Hewage AS, Hinton CV (2011) ROS enhances CXCR4-mediated functions through inactivation of PTEN in prostate cancer cells. Biochem Biophys Res Commun. doi:10.1016/j.bbrc.2011.05.074
Jiang F, Zhang Y, Dusting GJ (2011) NADPH oxidase-mediated redox signaling: roles in cellular stress response, stress tolerance, and tissue repair. Pharmacol rev 63(1):218–242. doi:10.1124/pr.110.002980
Fayard E, Tintignac LA, Baudry A, Hemmings BA (2005) Protein kinase B/Akt at a glance. J cell sci 118(Pt 24):5675–5678. doi:10.1242/jcs.02724
Carracedo A, Pandolfi PP (2008) The PTEN-PI3K pathway: of feedbacks and cross-talks. Oncogene 27(41):5527–5541. doi:10.1038/onc.2008.247
Kwon J, Lee SR, Yang KS, Ahn Y, Kim YJ, Stadtman ER, Rhee SG (2004) Reversible oxidation and inactivation of the tumor suppressor PTEN in cells stimulated with peptide growth factors. Proc Natl Acad Sci U S A 101(47):16419–16424. doi:10.1073/pnas.0407396101
Seo JH, Ahn Y, Lee SR, Yeol Yeo C, Chung Hur K (2005) The major target of the endogenously generated reactive oxygen species in response to insulin stimulation is phosphatase and tensin homolog and not phosphoinositide-3 kinase (PI-3 kinase) in the PI-3 kinase/Akt pathway. Mol biol cell 16(1):348–357. doi:10.1091/mbc.E04-05-0369
Dorfmuller P, Chaumais MC, Giannakouli M, Durand-Gasselin I, Raymond N, Fadel E, Mercier O, Charlotte F, Montani D, Simonneau G, Humbert M, Perros F Increased oxidative stress and severe arterial remodeling induced by permanent high-flow challenge in experimental pulmonary hypertension. Respir Res 12:119. doi: 10.1186/1465-9921-12-119
Lim SD, Sun C, Lambeth JD, Marshall F, Amin M, Chung L, Petros JA, Arnold RS (2005) Increased Nox1 and hydrogen peroxide in prostate cancer. Prostate 62(2):200–207. doi:10.1002/pros.20137
Brar SS, Corbin Z, Kennedy TP, Hemendinger R, Thornton L, Bommarius B, Arnold RS, Whorton AR, Sturrock AB, Huecksteadt TP, Quinn MT, Krenitsky K, Ardie KG, Lambeth JD, Hoidal JR (2003) NOX5 NAD(P)H oxidase regulates growth and apoptosis in DU 145 prostate cancer cells. Am J Physiol Cell Physiol 285(2):C353–C369. doi:10.1152/ajpcell.00525.2002
Wartenberg M, Hoffmann E, Schwindt H, Grunheck F, Petros J, Arnold JR, Hescheler J, Sauer H (2005) Reactive oxygen species-linked regulation of the multidrug resistance transporter P-glycoprotein in Nox-1 overexpressing prostate tumor spheroids. FEBS Lett 579(20):4541–4549. doi:10.1016/j.febslet.2005.06.078
Lu JP, Monardo L, Bryskin I, Hou ZF, Trachtenberg J, Wilson BC, Pinthus JH Androgens induce oxidative stress and radiation resistance in prostate cancer cells though NADPH oxidase. Prostate Cancer Prostatic Dis 13 (1):39–46. doi: 10.1038/pcan.2009.24
Woolley JF, Corcoran A, Groeger G, Landry WD, Cotter TG Redox-Regulated Growth Factor Survival Signaling. Antioxid Redox Signal. doi:10.1089/ars.2012.5028
Sundaresan M, Yu ZX, Ferrans VJ, Irani K, Finkel T (1995) Requirement for generation of H2O2 for platelet-derived growth factor signal transduction. Science 270(5234):296–299
Bae YS, Kang SW, Seo MS, Baines IC, Tekle E, Chock PB, Rhee SG (1997) Epidermal growth factor (EGF)-induced generation of hydrogen peroxide. Role in EGF receptor-mediated tyrosine phosphorylation. J biol chem 272(1):217–221
Suh YA, Arnold RS, Lassegue B, Shi J, Xu X, Sorescu D, Chung AB, Griendling KK, Lambeth JD (1999) Cell transformation by the superoxide-generating oxidase Mox1. Nature 401(6748):79–82. doi:10.1038/43459
Weyemi U, Redon CE, Parekh PR, Dupuy C, Bonner WM NADPH Oxidases NOXs and DUPXs As Putative Targets for Cancer Therapy. Anticancer Agents Med Chem
Finak G, Bertos N, Pepin F, Sadekova S, Souleimanova M, Zhao H, Chen H, Omeroglu G, Meterissian S, Omeroglu A, Hallett M, Park M (2008) Stromal gene expression predicts clinical outcome in breast cancer. Nat med 14(5):518–527. doi:10.1038/nm1764
Ginos MA, Page GP, Michalowicz BS, Patel KJ, Volker SE, Pambuccian SE, Ondrey FG, Adams GL, Gaffney PM (2004) Identification of a gene expression signature associated with recurrent disease in squamous cell carcinoma of the head and neck. Cancer res 64(1):55–63
Beroukhim R, Brunet JP, Di Napoli A, Mertz KD, Seeley A, Pires MM, Linhart D, Worrell RA, Moch H, Rubin MA, Sellers WR, Meyerson M, Linehan WM, Kaelin WG Jr, Signoretti S (2009) Patterns of gene expression and copy-number alterations in von-hippel lindau disease-associated and sporadic clear cell carcinoma of the kidney. Cancer res 69(11):4674–4681. doi:10.1158/0008-5472.CAN-09-0146
Alizadeh AA, Eisen MB, Davis RE, Ma C, Lossos IS, Rosenwald A, Boldrick JC, Sabet H, Tran T, Yu X, Powell JI, Yang L, Marti GE, Moore T, Hudson J Jr, Lu L, Lewis DB, Tibshirani R, Sherlock G, Chan WC, Greiner TC, Weisenburger DD, Armitage JO, Warnke R, Levy R, Wilson W, Grever MR, Byrd JC, Botstein D, Brown PO, Staudt LM (2000) Distinct types of diffuse large B-cell lymphoma identified by gene expression profiling. Nature 403(6769):503–511. doi:10.1038/35000501
Tomlins SA, Mehra R, Rhodes DR, Cao X, Wang L, Dhanasekaran SM, Kalyana-Sundaram S, Wei JT, Rubin MA, Pienta KJ, Shah RB, Chinnaiyan AM (2007) Integrative molecular concept modeling of prostate cancer progression. Nat genet 39(1):41–51. doi:10.1038/ng1935
Acknowledgments
We thank Dr. Jaideep Chaudhary for assistance in database mining. We also thank Dr. Ritu Aneja, Dr. Ayesha Don-Salu-Hewage and Vanessa L. Adams for technical assistance.
Grant Support
Research in this laboratory is supported, in part, by National Institutes of Health grants 2R25GM060414 (KJJ and ASDSH), P201MD002285 (CVH and VLA), F31CA153908 (MAC), and 8G12MD007590 (CVH), the American Association for the Advancement of Science (AAAS) Women’s International Research Collaborations (WIRC) for Minority Serving Institutions (MSIs), a National Science Foundation grant, and the United Negro College Fund-Henry C. McBay Fellowship.
Conflict of Interest
The authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Jones, K.J., Chetram, M.A., Bethea, D.A. et al. Cysteine (C)-X-C Receptor 4 Regulates NADPH Oxidase-2 During Oxidative Stress in Prostate Cancer Cells. Cancer Microenvironment 6, 277–288 (2013). https://doi.org/10.1007/s12307-013-0136-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12307-013-0136-0