Abstract
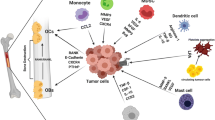
Bone serves one of the most congenial metastatic microenvironments for multiple types of solid tumors, but its role in this process remains under-explored. Among many cell populations constituting the bone and bone marrow microenvironment, osteoblasts (originated from mesenchymal stem cells) and osteoclasts (originated from hematopoietic stem cells) have been the main research focus for pro-tumorigenic roles. Recently, increasing evidence further elucidates that hematopoietic lineage cells as well as stromal cells in the bone marrow mediate distinct but critical functions in tumor growth, metastasis, angiogenesis and apoptosis in the bone microenvironment. This review article summarizes the key evidence describing differential roles of bone marrow cells, including hematopoietic stem cells (HSCs), megakaryocytes, macrophages and myeloid-derived suppressor cells in the development of metastatic bone lesions. HSCs promote tumor growth by switching on angiogenesis, but at the same time compete with metastatic tumor cells for occupancy of osteoblastic niche. Megakaryocytes negatively regulate the extravasating tumor cells by inducing apoptosis and suppressing proliferation. Macrophages and myeloid cells have pro-tumorigenic roles in general, suggesting a similar effect in the bone marrow. Hematopoietic and stromal cell populations in the bone marrow, previously considered as simple by-standers in the context of tumor metastasis, have distinct and active roles in promoting or suppressing tumor growth and metastasis in bone. Further investigation on the extended roles of bone marrow cells will help formulate better approaches to treatment through improved understanding of the metastatic bone microenvironment.

Similar content being viewed by others
References
Fidler IJ (2003) The pathogenesis of cancer metastasis: the ‘seed and soil’ hypothesis revisited. Nat Rev Canc 3(6):453–458
Talmadge JE, Fidler IJ (2010) AACR centennial series: the biology of cancer metastasis: historical perspective. Canc Res 70(14):5649–5669. doi:10.1158/0008-5472.CAN-10-1040
Fidler IJ (2002) Critical determinants of metastasis. Semin Canc Biol 12(2):89–96. doi:10.1006/scbi.2001.0416
Paget S (1989) The distribution of secondary growths in cancer of the breast. 1889. Canc Metastasis Rev 8(2):98–101
Ewing J (1928) Neoplastic diseases; a treatise on tumors. 3d ed rev. and enl., with 546 illustrations. edn. W.B. Saunders, Philadelphia, London
Geldof AA (1997) Models for cancer skeletal metastasis: a reappraisal of Batson’s plexus. Anticancer Res 17(3A):1535–1539
Fidler IJ, Kripke ML (1977) Metastasis results from preexisting variant cells within a malignant tumor. Science 197(4306):893–895
Hart IR, Fidler IJ (1980) Role of organ selectivity in the determination of metastatic patterns of B16 melanoma. Canc Res 40(7):2281–2287
Tarin D, Price JE, Kettlewell MG, Souter RG, Vass AC, Crossley B (1984) Clinicopathological observations on metastasis in man studied in patients treated with peritoneovenous shunts. Br Med J (Clin Res Ed) 288(6419):749–751
Tarin D, Price JE, Kettlewell MG, Souter RG, Vass AC, Crossley B (1984) Mechanisms of human tumor metastasis studied in patients with peritoneovenous shunts. Canc Res 44(8):3584–3592
Hess KR, Varadhachary GR, Taylor SH, Wei W, Raber MN, Lenzi R, Abbruzzese JL (2006) Metastatic patterns in adenocarcinoma. Cancer 106(7):1624–1633. doi:10.1002/cncr.21778
Bubendorf L, Schopfer A, Wagner U, Sauter G, Moch H, Willi N, Gasser TC, Mihatsch MJ (2000) Metastatic patterns of prostate cancer: an autopsy study of 1,589 patients. Hum Pathol 31(5):578–583
Mundy GR (2002) Metastasis to bone: causes, consequences and therapeutic opportunities. Nat Rev Canc 2(8):584–593. doi:10.1038/nrc867
Roodman GD (2004) Mechanisms of bone metastasis. New Engl J Med 350(16):1655–1664. doi:10.1056/NEJMra030831
Rubin MA, Putzi M, Mucci N, Smith DC, Wojno K, Korenchuk S, Pienta KJ (2000) Rapid (“warm”) autopsy study for procurement of metastatic prostate cancer. Clin Canc Res 6(3):1038–1045
Weilbaecher KN, Guise TA, McCauley LK (2011) Cancer to bone: a fatal attraction. Nat Rev Canc 11(6):411–425. doi:10.1038/nrc3055
Casimiro S, Guise TA, Chirgwin J (2009) The critical role of the bone microenvironment in cancer metastases. Mol Cell Endocrinol 310(1–2):71–81. doi:10.1016/j.mce.2009.07.004
Guise TA, Yin JJ, Taylor SD, Kumagai Y, Dallas M, Boyce BF, Yoneda T, Mundy GR (1996) Evidence for a causal role of parathyroid hormone-related protein in the pathogenesis of human breast cancer-mediated osteolysis. J Clin Investig 98(7):1544–1549. doi:10.1172/JCI118947
Yin JJ, Selander K, Chirgwin JM, Dallas M, Grubbs BG, Wieser R, Massague J, Mundy GR, Guise TA (1999) TGF-beta signaling blockade inhibits PTHrP secretion by breast cancer cells and bone metastases development. J Clin Investig 103(2):197–206. doi:10.1172/JCI3523
Li X, Loberg R, Liao J, Ying C, Snyder LA, Pienta KJ, McCauley LK (2009) A destructive cascade mediated by CCL2 facilitates prostate cancer growth in bone. Canc Res 69(4):1685–1692. doi:10.1158/0008-5472.CAN-08-2164
Liao J, Li X, Koh AJ, Berry JE, Thudi N, Rosol TJ, Pienta KJ, McCauley LK (2008) Tumor expressed PTHrP facilitates prostate cancer-induced osteoblastic lesions. Int J Canc 123(10):2267–2278. doi:10.1002/ijc.23602
Liao J, McCauley LK (2006) Skeletal metastasis: established and emerging roles of parathyroid hormone related protein (PTHrP). Canc Metastasis Rev 25(4):559–571. doi:10.1007/s10555-006-9033-z
Bergfeld SA, DeClerck YA (2010) Bone marrow-derived mesenchymal stem cells and the tumor microenvironment. Canc Metastasis Rev 29(2):249–261. doi:10.1007/s10555-010-9222-7
Ara T, Declerck YA (2010) Interleukin-6 in bone metastasis and cancer progression. Eur J Canc 46(7):1223–1231. doi:10.1016/j.ejca.2010.02.026
Paul WE (1999) Fundamental immunology, 4th edn. Lippincott-Raven, Philadelphia
Schermer S (1967) The blood morphology of laboratory animals, 3rd edn. Davis, Philadelphia
Travlos GS (2006) Normal structure, function, and histology of the bone marrow. Toxicol Pathol 34(5):548–565. doi:10.1080/01926230600939856
Shirota T, Tavassoli M (1991) Cyclophosphamide-induced alterations of bone marrow endothelium: implications in homing of marrow cells after transplantation. Exp Hematol 19(5):369–373
Shirota T, Tavassoli M (1992) Alterations of bone marrow sinus endothelium induced by ionizing irradiation: implications in the homing of intravenously transplanted marrow cells. Blood Cell 18(2):197–214
Weiss L, Geduldig U (1991) Barrier cells: stromal regulation of hematopoiesis and blood cell release in normal and stressed murine bone marrow. Blood 78(4):975–990
Tavassoli M, Hardy CL (1990) Molecular basis of homing of intravenously transplanted stem cells to the marrow. Blood 76(6):1059–1070
Mazo IB, Gutierrez-Ramos JC, Frenette PS, Hynes RO, Wagner DD, von Andrian UH (1998) Hematopoietic progenitor cell rolling in bone marrow microvessels: parallel contributions by endothelial selectins and vascular cell adhesion molecule 1. J Exp Med 188(3):465–474
Frenette PS, Subbarao S, Mazo IB, von Andrian UH, Wagner DD (1998) Endothelial selectins and vascular cell adhesion molecule-1 promote hematopoietic progenitor homing to bone marrow. Proc Natl Acad Sci U S A 95(24):14423–14428
Peled A, Petit I, Kollet O, Magid M, Ponomaryov T, Byk T, Nagler A, Ben-Hur H, Many A, Shultz L, Lider O, Alon R, Zipori D, Lapidot T (1999) Dependence of human stem cell engraftment and repopulation of NOD/SCID mice on CXCR4. Science 283(5403):845–848
Muller A, Homey B, Soto H, Ge N, Catron D, Buchanan ME, McClanahan T, Murphy E, Yuan W, Wagner SN, Barrera JL, Mohar A, Verastegui E, Zlotnik A (2001) Involvement of chemokine receptors in breast cancer metastasis. Nature 410(6824):50–56. doi:10.1038/35065016
Taichman RS, Cooper C, Keller ET, Pienta KJ, Taichman NS, McCauley LK (2002) Use of the stromal cell-derived factor-1/CXCR4 pathway in prostate cancer metastasis to bone. Canc Res 62(6):1832–1837
Sun X, Cheng G, Hao M, Zheng J, Zhou X, Zhang J, Taichman RS, Pienta KJ, Wang J (2010) CXCL12/CXCR4/CXCR7 chemokine axis and cancer progression. Canc Metastasis Rev 29(4):709–722. doi:10.1007/s10555-010-9256-x
Jung Y, Wang J, Song J, Shiozawa Y, Havens A, Wang Z, Sun YX, Emerson SG, Krebsbach PH, Taichman RS (2007) Annexin II expressed by osteoblasts and endothelial cells regulates stem cell adhesion, homing, and engraftment following transplantation. Blood 110(1):82–90. doi:10.1182/blood-2006-05-021352
Shiozawa Y, Havens AM, Jung Y, Ziegler AM, Pedersen EA, Wang J, Lu G, Roodman GD, Loberg RD, Pienta KJ, Taichman RS (2008) Annexin II/annexin II receptor axis regulates adhesion, migration, homing, and growth of prostate cancer. J Cell Biochem 105(2):370–380. doi:10.1002/jcb.21835
Shiozawa Y, Pedersen EA, Havens AM, Jung Y, Mishra A, Joseph J, Kim JK, Patel LR, Ying C, Ziegler AM, Pienta MJ, Song J, Wang J, Loberg RD, Krebsbach PH, Pienta KJ, Taichman RS (2011) Human prostate cancer metastases target the hematopoietic stem cell niche to establish footholds in mouse bone marrow. J Clin Investig. doi:10.1172/JCI43414
Okamoto R, Ueno M, Yamada Y, Takahashi N, Sano H, Suda T, Takakura N (2005) Hematopoietic cells regulate the angiogenic switch during tumorigenesis. Blood 105(7):2757–2763. doi:10.1182/blood-2004-08-3317
Schneider A, Kalikin LM, Mattos AC, Keller ET, Allen MJ, Pienta KJ, McCauley LK (2005) Bone turnover mediates preferential localization of prostate cancer in the skeleton. Endocrinology 146(4):1727–1736. doi:10.1210/en.2004-1211
Lichtman MA, Chamberlain JK, Simon W, Santillo PA (1978) Parasinusoidal location of megakaryocytes in marrow: a determinant of platelet release. Am J Hematol 4(4):303–312
Li X, Koh AJ, Wang Z, Soki FN, Park SI, Pienta KJ, McCauley LK (2011) Inhibitory effects of megakaryocytic cells in prostate cancer skeletal metastasis. J Bone Miner Res 26(1):125–134. doi:10.1002/jbmr.204
Kacena MA, Gundberg CM, Horowitz MC (2006) A reciprocal regulatory interaction between megakaryocytes, bone cells, and hematopoietic stem cells. Bone 39(5):978–984. doi:10.1016/j.bone.2006.05.019
Kacena MA, Nelson T, Clough ME, Lee SK, Lorenzo JA, Gundberg CM, Horowitz MC (2006) Megakaryocyte-mediated inhibition of osteoclast development. Bone 39(5):991–999. doi:10.1016/j.bone.2006.05.004
Kacena MA, Shivdasani RA, Wilson K, Xi Y, Troiano N, Nazarian A, Gundberg CM, Bouxsein ML, Lorenzo JA, Horowitz MC (2004) Megakaryocyte-osteoblast interaction revealed in mice deficient in transcription factors GATA-1 and NF-E2. J Bone Miner Res 19(4):652–660. doi:10.1359/JBMR.0301254
Bord S, Frith E, Ireland DC, Scott MA, Craig JI, Compston JE (2005) Megakaryocytes modulate osteoblast synthesis of type-l collagen, osteoprotegerin, and RANKL. Bone 36(5):812–819. doi:10.1016/j.bone.2004.12.006
Calvi LM, Adams GB, Weibrecht KW, Weber JM, Olson DP, Knight MC, Martin RP, Schipani E, Divieti P, Bringhurst FR, Milner LA, Kronenberg HM, Scadden DT (2003) Osteoblastic cells regulate the haematopoietic stem cell niche. Nature 425(6960):841–846. doi:10.1038/nature02040
Udagawa N, Takahashi N, Jimi E, Matsuzaki K, Tsurukai T, Itoh K, Nakagawa N, Yasuda H, Goto M, Tsuda E, Higashio K, Gillespie MT, Martin TJ, Suda T (1999) Osteoblasts/stromal cells stimulate osteoclast activation through expression of osteoclast differentiation factor/RANKL but not macrophage colony-stimulating factor: receptor activator of NF-kappa B ligand. Bone 25(5):517–523
Ahmed N, Khokher MA, Hassan HT (1999) Cytokine-induced expansion of human CD34+ stem/progenitor and CD34 + CD41+ early megakaryocytic marrow cells cultured on normal osteoblasts. Stem Cell 17(2):92–99. doi:10.1002/stem.170092
Roato I, D'Amelio P, Gorassini E, Grimaldi A, Bonello L, Fiori C, Delsedime L, Tizzani A, De Libero A, Isaia G, Ferracini R (2008) Osteoclasts are active in bone forming metastases of prostate cancer patients. PLoS One 3(11):e3627. doi:10.1371/journal.pone.0003627
Carmeliet P, Jain RK (2011) Molecular mechanisms and clinical applications of angiogenesis. Nature 473(7347):298–307. doi:10.1038/nature10144
Nieswandt B, Hafner M, Echtenacher B, Mannel DN (1999) Lysis of tumor cells by natural killer cells in mice is impeded by platelets. Canc Res 59(6):1295–1300
Boucharaba A, Serre CM, Gres S, Saulnier-Blache JS, Bordet JC, Guglielmi J, Clezardin P, Peyruchaud O (2004) Platelet-derived lysophosphatidic acid supports the progression of osteolytic bone metastases in breast cancer. J Clin Investig 114(12):1714–1725. doi:10.1172/JCI22123
Gupta GP, Massague J (2004) Platelets and metastasis revisited: a novel fatty link. J Clin Investig 114(12):1691–1693. doi:10.1172/JCI23823
Mizutani K, Sud S, McGregor NA, Martinovski G, Rice BT, Craig MJ, Varsos ZS, Roca H, Pienta KJ (2009) The chemokine CCL2 increases prostate tumor growth and bone metastasis through macrophage and osteoclast recruitment. Neoplasia 11(11):1235–1242
Loberg RD, Ying C, Craig M, Yan L, Snyder LA, Pienta KJ (2007) CCL2 as an important mediator of prostate cancer growth in vivo through the regulation of macrophage infiltration. Neoplasia 9(7):556–562
Halin S, Rudolfsson SH, Van Rooijen N, Bergh A (2009) Extratumoral macrophages promote tumor and vascular growth in an orthotopic rat prostate tumor model. Neoplasia 11(2):177–186
Solinas G, Germano G, Mantovani A, Allavena P (2009) Tumor-associated macrophages (TAM) as major players of the cancer-related inflammation. J Leukoc Biol 86(5):1065–1073. doi:10.1189/jlb.0609385
Hiraoka K, Zenmyo M, Watari K, Iguchi H, Fotovati A, Kimura YN, Hosoi F, Shoda T, Nagata K, Osada H, Ono M, Kuwano M (2008) Inhibition of bone and muscle metastases of lung cancer cells by a decrease in the number of monocytes/macrophages. Canc Sci 99(8):1595–1602. doi:10.1111/j.1349-7006.2008.00880.x
Bingle L, Brown NJ, Lewis CE (2002) The role of tumour-associated macrophages in tumour progression: implications for new anticancer therapies. J Pathol 196(3):254–265. doi:10.1002/path.1027
Sica A, Larghi P, Mancino A, Rubino L, Porta C, Totaro MG, Rimoldi M, Biswas SK, Allavena P, Mantovani A (2008) Macrophage polarization in tumour progression. Semin Canc Biol 18(5):349–355. doi:10.1016/j.semcancer.2008.03.004
Balkwill F, Mantovani A (2001) Inflammation and cancer: back to Virchow? Lancet 357(9255):539–545. doi:10.1016/S0140-6736(00)04046-0
Loberg RD, Ying C, Craig M, Day LL, Sargent E, Neeley C, Wojno K, Snyder LA, Yan L, Pienta KJ (2007) Targeting CCL2 with systemic delivery of neutralizing antibodies induces prostate cancer tumor regression in vivo. Canc Res 67(19):9417–9424. doi:10.1158/0008-5472.CAN-07-1286
Roca H, Varsos Z, Pienta KJ (2008) CCL2 protects prostate cancer PC3 cells from autophagic death via phosphatidylinositol 3-kinase/AKT-dependent survivin up-regulation. J Biol Chem 283(36):25057–25073. doi:10.1074/jbc.M801073200
Roca H, Varsos ZS, Sud S, Craig MJ, Ying C, Pienta KJ (2009) CCL2 and interleukin-6 promote survival of human CD11b + peripheral blood mononuclear cells and induce M2-type macrophage polarization. J Biol Chem 284(49):34342–34354. doi:10.1074/jbc.M109.042671
Zhang J, Lu Y, Pienta KJ (2010) Multiple roles of chemokine (C-C motif) ligand 2 in promoting prostate cancer growth. J Natl Canc Inst 102(8):522–528. doi:10.1093/jnci/djq044
Lin EY, Li JF, Gnatovskiy L, Deng Y, Zhu L, Grzesik DA, Qian H, Xue XN, Pollard JW (2006) Macrophages regulate the angiogenic switch in a mouse model of breast cancer. Canc Res 66(23):11238–11246. doi:10.1158/0008-5472.CAN-06-1278
Leek RD, Lewis CE, Whitehouse R, Greenall M, Clarke J, Harris AL (1996) Association of macrophage infiltration with angiogenesis and prognosis in invasive breast carcinoma. Canc Res 56(20):4625–4629
Hildenbrand R, Dilger I, Horlin A, Stutte HJ (1995) Urokinase and macrophages in tumour angiogenesis. Br J Canc 72(4):818–823
Miles DW, Happerfield LC, Naylor MS, Bobrow LG, Rubens RD, Balkwill FR (1994) Expression of tumour necrosis factor (TNF alpha) and its receptors in benign and malignant breast tissue. Int J Canc 56(6):777–782
Jung YJ, Isaacs JS, Lee S, Trepel J, Neckers L (2003) IL-1beta-mediated up-regulation of HIF-1alpha via an NFkappaB/COX-2 pathway identifies HIF-1 as a critical link between inflammation and oncogenesis. FASEB J 17(14):2115–2117. doi:10.1096/fj.03-0329fje
MacMicking J, Xie QW, Nathan C (1997) Nitric oxide and macrophage function. Annu Rev Immunol 15:323–350. doi:10.1146/annurev.immunol.15.1.323
Pollard JW (2004) Tumour-educated macrophages promote tumour progression and metastasis. Nat Rev Canc 4(1):71–78. doi:10.1038/nrc1256
Qian BZ, Pollard JW (2010) Macrophage diversity enhances tumor progression and metastasis. Cell 141(1):39–51. doi:10.1016/j.cell.2010.03.014
Lewis CE, Pollard JW (2006) Distinct role of macrophages in different tumor microenvironments. Canc Res 66(2):605–612. doi:10.1158/0008-5472.CAN-05-4005
Alexander K, Chang M, Maylin E, Kohler T, Muller R, Wu A, Van Rooijen N, Sweet M, Hume D, Raggatt L, Pettit A (2011) Osteal macrophages promote in vivo intramembranous bone healing in a mouse tibial injury model. J Bone Miner Res 26(7):1517–1532. doi:10.1002/jbmr.354
Chang MK, Raggatt LJ, Alexander KA, Kuliwaba JS, Fazzalari NL, Schroder K, Maylin ER, Ripoll VM, Hume DA, Pettit AR (2008) Osteal tissue macrophages are intercalated throughout human and mouse bone lining tissues and regulate osteoblast function in vitro and in vivo. J Immunol 181(2):1232–1244
Dvorak HF (1986) Tumors: wounds that do not heal. Similarities between tumor stroma generation and wound healing. New Engl J Med 315(26):1650–1659. doi:10.1056/NEJM198612253152606
Zumsteg A, Christofori G (2009) Corrupt policemen: inflammatory cells promote tumor angiogenesis. Curr Opin Oncol 21(1):60–70. doi:10.1097/CCO.0b013e32831bed7e
Murdoch C, Muthana M, Coffelt SB, Lewis CE (2008) The role of myeloid cells in the promotion of tumour angiogenesis. Nat Rev Canc 8(8):618–631. doi:10.1038/nrc2444
Coffelt SB, Lewis CE, Naldini L, Brown JM, Ferrara N, De Palma M (2010) Elusive identities and overlapping phenotypes of proangiogenic myeloid cells in tumors. Am J Pathol 176(4):1564–1576. doi:10.2353/ajpath.2010.090786
Nagaraj S, Gupta K, Pisarev V, Kinarsky L, Sherman S, Kang L, Herber DL, Schneck J, Gabrilovich DI (2007) Altered recognition of antigen is a mechanism of CD8+ T cell tolerance in cancer. Nat Med 13(7):828–835. doi:10.1038/nm1609
Gabrilovich DI, Nagaraj S (2009) Myeloid-derived suppressor cells as regulators of the immune system. Nat Rev Immunol 9(3):162–174. doi:10.1038/nri2506
Nagaraj S, Gabrilovich DI (2008) Tumor escape mechanism governed by myeloid-derived suppressor cells. Canc Res 68(8):2561–2563. doi:10.1158/0008-5472.CAN-07-6229
Yang L, DeBusk LM, Fukuda K, Fingleton B, Green-Jarvis B, Shyr Y, Matrisian LM, Carbone DP, Lin PC (2004) Expansion of myeloid immune suppressor Gr + CD11b + cells in tumor-bearing host directly promotes tumor angiogenesis. Canc Cell 6(4):409–421. doi:10.1016/j.ccr.2004.08.031
Yang L, Edwards CM, Mundy GR (2010) Gr-1 + CD11b + myeloid-derived suppressor cells (MDSCs): formidable partners in tumor metastasis. J Bone Miner Res 25(8):1701–1706. doi:10.1002/jbmr.154
Kim SJ, Kim JS, Papadopoulos J, Wook Kim S, Maya M, Zhang F, He J, Fan D, Langley R, Fidler IJ (2009) Circulating monocytes expressing CD31: implications for acute and chronic angiogenesis. Am J Pathol 174(5):1972–1980. doi:10.2353/ajpath.2009.080819
Shojaei F, Zhong C, Wu X, Yu L, Ferrara N (2008) Role of myeloid cells in tumor angiogenesis and growth. Trends Cell Biol 18(8):372–378. doi:10.1016/j.tcb.2008.06.003
Ahn GO, Brown JM (2008) Matrix metalloproteinase-9 is required for tumor vasculogenesis but not for angiogenesis: role of bone marrow-derived myelomonocytic cells. Canc Cell 13(3):193–205. doi:10.1016/j.ccr.2007.11.032
Taichman NS, Young S, Cruchley AT, Taylor P, Paleolog E (1997) Human neutrophils secrete vascular endothelial growth factor. J Leukoc Biol 62(3):397–400
Yang L, Huang J, Ren X, Gorska AE, Chytil A, Aakre M, Carbone DP, Matrisian LM, Richmond A, Lin PC, Moses HL (2008) Abrogation of TGF beta signaling in mammary carcinomas recruits Gr-1 + CD11b + myeloid cells that promote metastasis. Canc Cell 13(1):23–35. doi:10.1016/j.ccr.2007.12.004
ASBMR 29th Annual Meeting (2007) J Bone Miner Res 22(S1):s2–s51. doi:10.1002/jbmr.5650221402
Zhuang J, Yang L, Lwin ST, Edwards CM, Edwards JR, Mundy GR (2008) Osteoclasts in myeloma are derived from Gr-1 + CD11b + myeloid immune suppressor cells of the bone marrow niche in vivo. ASH Annu Meet Abstr 112(11):736
Melani C, Sangaletti S, Barazzetta FM, Werb Z, Colombo MP (2007) Amino-biphosphonate-mediated MMP-9 inhibition breaks the tumor-bone marrow axis responsible for myeloid-derived suppressor cell expansion and macrophage infiltration in tumor stroma. Canc Res 67(23):11438–11446. doi:10.1158/0008-5472.CAN-07-1882
Shiozawa Y, Jung Y, Ziegler AM, Pedersen EA, Wang J, Wang Z, Song J, Lee CH, Sud S, Pienta KJ, Krebsbach PH, Taichman RS (2010) Erythropoietin couples hematopoiesis with bone formation. PLoS One 5(5):e10853. doi:10.1371/journal.pone.0010853
Naveiras O, Nardi V, Wenzel PL, Hauschka PV, Fahey F, Daley GQ (2009) Bone-marrow adipocytes as negative regulators of the haematopoietic microenvironment. Nature 460(7252):259–263. doi:10.1038/nature08099
Kaplan RN, Psaila B, Lyden D (2006) Bone marrow cells in the ‘pre-metastatic niche’: within bone and beyond. Canc Metastasis Rev 25(4):521–529. doi:10.1007/s10555-006-9036-9
Kaplan RN, Riba RD, Zacharoulis S, Bramley AH, Vincent L, Costa C, MacDonald DD, Jin DK, Shido K, Kerns SA, Zhu Z, Hicklin D, Wu Y, Port JL, Altorki N, Port ER, Ruggero D, Shmelkov SV, Jensen KK, Rafii S, Lyden D (2005) VEGFR1-positive haematopoietic bone marrow progenitors initiate the pre-metastatic niche. Nature 438(7069):820–827. doi:10.1038/nature04186
Acknowledgements
This work was financially supported by the Department of Defense Prostate Cancer Research Program Grants W81XWH-10-1-0546 (Serk In Park) and W81XWH-08-1-0037 (Laurie K. McCauley); and the National Cancer Institute Program Project Grant P01CA093900 (Laurie K. McCauley). The authors thank Janice E. Berry, Amy J. Koh and Matthew Eber for their assistance with preparation of this manuscript.
Conflict of Interest
The authors declare no financial conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Park, S.I., Soki, F.N. & McCauley, L.K. Roles of Bone Marrow Cells in Skeletal Metastases: No Longer Bystanders. Cancer Microenvironment 4, 237–246 (2011). https://doi.org/10.1007/s12307-011-0081-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12307-011-0081-8