Abstract
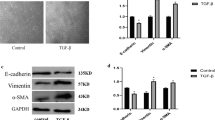
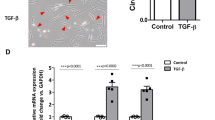
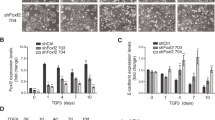
Epithelial to mesenchymal transition (EMT) is a process by which an epithelial cell alters its phenotype to that of a mesenchymal cell and plays a critical role in embryonic development, tumour invasion and metastasis and tissue fibrosis. Transforming growth factor-β1 (TGF-β1) continues to be regarded as the key growth factor involved in driving EMT however recently tumour necrosis factor α (TNFα) has been demonstrated to accentuate TGF-β1 driven EMT. In this study we investigate how various signalling pathways contribute to this accentuated effect. A549 cells were treated with TGF-β1 (10 ng/ml), TNFα (20 ng/ml) or a combination of both for 72 h and EMT assessed. The effect of selective inhibition of the SMAD, MAPK and NF-κB pathways on EMT was assessed. A549 cells treated with TGF-β1 downregulate the expression of epithelial markers, increase the expression of mesenchymal markers, secrete matrix-metalloproteinases and become invasive. Significantly, TGF-β1 driven EMT is accentuated by co-treatment with TNFα. SMAD 3 inhibition attenuated TGF-β1 driven EMT but has no effect on the accentuation effect of TNFα. However, inhibiting IKKβ blocked both TGF-β1 driven EMT and the accentuating action of TNFα. Inhibiting p38 and ERK signalling had no effect on EMT. TNFα accentuates TGF-β1 driven EMT in A549 cells via a SMAD 2/3 independent mechanism involving the NF-κB pathway independent of p38 and ERK 1/2 activation.






Similar content being viewed by others
References
Thiery JP, Acloque H, Huang RY, Nieto MA (2009) Epithelial-mesenchymal transitions in development and disease. Cell 139(5):871–890
Jain R, Shaul PW, Borok Z, Willis BC (2007) Endothelin-1 induces alveolar epithelial-mesenchymal transition through endothelin type A receptor-mediated production of TGF-beta1. Am J Respir Cell Mol Biol 37(1):38–47
Borthwick LA, Parker SM, Brougham KA, Johnson GE, Gorowiec MR, Ward C, Lordan JL, Corris PA, Kirby JA, Fisher AJ (2009) Epithelial to mesenchymal transition (EMT) and airway remodelling after human lung transplantation. Thorax 64(9):770–777
Kalluri R, Neilson EG (2003) Epithelial-mesenchymal transition and its implications for fibrosis. J Clin Invest 112(12):1776–1784
Pagan R, Martin I, Llobera M, Vilaro S (1997) Epithelial-mesenchymal transition of cultured rat neonatal hepatocytes is differentially regulated in response to epidermal growth factor and dimethyl sulfoxide. Hepatology (Baltimore, Md) 25(3):598–606
Ahmed N, Maines-Bandiera S, Quinn MA, Unger WG, Dedhar S, Auersperg N (2006) Molecular pathways regulating EGF-induced epithelio-mesenchymal transition in human ovarian surface epithelium. Am J Physiol 290(6):C1532–C1542
Yang J, Dai C, Liu Y (2005) A novel mechanism by which hepatocyte growth factor blocks tubular epithelial to mesenchymal transition. J Am Soc Nephrol 16(1):68–78
Strutz F, Zeisberg M, Ziyadeh FN, Yang CQ, Kalluri R, Muller GA, Neilson EG (2002) Role of basic fibroblast growth factor-2 in epithelial-mesenchymal transformation. Kidney Int 61(5):1714–1728
Kelly M, Kolb M, Bonniaud P, Gauldie J (2003) Re-evaluation of fibrogenic cytokines in lung fibrosis. Curr Pharm Des 9(1):39–49
Flanders KC (2004) Smad3 as a mediator of the fibrotic response. Int J Exp Pathol 85(2):47–64
Derynck R, Zhang YE (2003) Smad-dependent and Smad-independent pathways in TGF-beta family signalling. Nature 425(6958):577–584
Valcourt U, Kowanetz M, Niimi H, Heldin CH, Moustakas A (2005) TGF-beta and the Smad signaling pathway support transcriptomic reprogramming during epithelial-mesenchymal cell transition. Mol Biol Cell 16(4):1987–2002
Moustakas A, Heldin CH (2005) Non-Smad TGF-beta signals. J Cell Sci 118(Pt 16):3573–3584
Javelaud D, Mauviel A (2005) Crosstalk mechanisms between the mitogen-activated protein kinase pathways and Smad signaling downstream of TGF-beta: implications for carcinogenesis. Oncogene 24(37):5742–5750
Chuang MJ, Sun KH, Tang SJ, Deng MW, Wu YH, Sung JS, Cha TL, Sun GH (2008) Tumor-derived tumor necrosis factor-alpha promotes progression and epithelial-mesenchymal transition in renal cell carcinoma cells. Cancer Sci 99(5):905–913
Grund EM, Kagan D, Tran CA, Zeitvogel A, Starzinski-Powitz A, Nataraja S, Palmer SS (2008) Tumor necrosis factor-alpha regulates inflammatory and mesenchymal responses via mitogen-activated protein kinase kinase, p38, and nuclear factor kappaB in human endometriotic epithelial cells. Mol Pharmacol 73(5):1394–1404
Camara J, Jarai G. Epithelial-mesenchymal transition in primary human bronchial epithelial cells is Smad-dependent and enhanced by fibronectin and TNF-alpha. Fibrogenesis Tissue Repair 3(1):2
Kasai H, Allen JT, Mason RM, Kamimura T, Zhang Z (2005) TGF-beta1 induces human alveolar epithelial to mesenchymal cell transition (EMT). Respir Res 6:56
Yamauchi Y, Kohyama T, Takizawa H, Kamitani S, Desaki M, Takami K, Kawasaki S, Kato J, Nagase T. Tumor necrosis factor-alpha enhances both epithelial-mesenchymal transition and cell contraction induced in A549 human alveolar epithelial cells by transforming growth factor-beta1. Exp Lung Res 36(1):12–24
Liu X (2008) Inflammatory cytokines augments TGF-beta1-induced epithelial-mesenchymal transition in A549 cells by up-regulating TbetaR-I. Cell Motil Cytoskeleton 65(12):935–944
Borthwick LA, McIlroy EI, Gorowiec MR, Brodlie M, Johnson GE, Ward C, Lordan JL, Corris PA, Kirby JA, Fisher AJ. Inflammation and epithelial to mesenchymal transition in lung transplant recipients: role in dysregulated epithelial wound repair. Am J Transplant 10(3):498–509
Bates RC, Mercurio AM (2003) Tumor necrosis factor-alpha stimulates the epithelial-to-mesenchymal transition of human colonic organoids. Mol Biol Cell 14(5):1790–1800
Borthwick LA, Sunny SS, Oliphant V, Perry J, Brodlie M, Johnson GE, Ward C, Gould K, Corris PA, De Soyza A, Fisher AJ. Pseudomonas aeruginosa accentuates epithelial to mesenchymal transition in the airway. Eur Respir J
De Soyza A, Ellis CD, Khan CM, Corris PA, Demarco de Hormaeche R (2004) Burkholderia cenocepacia lipopolysaccharide, lipid A, and proinflammatory activity. Am J Respir Crit Care Med 170(1):70–77
Yu L, Hebert MC, Zhang YE (2002) TGF-beta receptor-activated p38 MAP kinase mediates Smad-independent TGF-beta responses. EMBO J 21(14):3749–3759
Vietor I, Schwenger P, Li W, Schlessinger J, Vilcek J (1993) Tumor necrosis factor-induced activation and increased tyrosine phosphorylation of mitogen-activated protein (MAP) kinase in human fibroblasts. J Biol Chem 268(25):18994–18999
Saile B, Matthes N, El Armouche H, Neubauer K, Ramadori G (2001) The bcl, NFkappaB and p53/p21WAF1 systems are involved in spontaneous apoptosis and in the anti-apoptotic effect of TGF-beta or TNF-alpha on activated hepatic stellate cells. Eur J Cell Biol 80(8):554–561
Gingery A, Bradley EW, Pederson L, Ruan M, Horwood NJ, Oursler MJ (2008) TGF-beta coordinately activates TAK1/MEK/AKT/NFkB and SMAD pathways to promote osteoclast survival. Exp Cell Res 314(15):2725–2738
Mosser DM, Edwards JP (2008) Exploring the full spectrum of macrophage activation. Nat Rev 8(12):958–969
Armendariz-Borunda J, Katayama K, Seyer JM (1992) Transcriptional mechanisms of type I collagen gene expression are differentially regulated by interleukin-1 beta, tumor necrosis factor alpha, and transforming growth factor beta in Ito cells. J Biol Chem 267(20):14316–14321
Regan MC, Kirk SJ, Hurson M, Sodeyama M, Wasserkrug HL, Barbul A (1993) Tumor necrosis factor-alpha inhibits in vivo collagen synthesis. Surgery 113(2):173–177
Solis-Herruzo JA, Brenner DA, Chojkier M (1988) Tumor necrosis factor alpha inhibits collagen gene transcription and collagen synthesis in cultured human fibroblasts. J Biol Chem 263(12):5841–5845
Tsujiuchi T, Sasaki Y, Murata N, Tsutsumi M, Nakae D, Konishi Y (2001) Elevated expression of transforming growth factor betas and the tumor necrosis factor family in lung adenocarcinomas induced by N-nitrosobis(2-hydroxypropyl)amine in rats. Exp Toxicol Pathol 53(4):291–295
Li R, Ruttinger D, Li R, Si LS, Wang YL (2003) Analysis of the immunological microenvironment at the tumor site in patients with non-small cell lung cancer. Langenbeck’s archives of surgery / Deutsche Gesellschaft fur Chirurgie 388(6):406–412
Saji H, Nakamura H, Awut I, Kawasaki N, Hagiwara M, Ogata A, Hosaka M, Saijo T, Kato Y, Kato H (2003) Significance of expression of TGF-beta in pulmonary metastasis in non-small cell lung cancer tissues. Ann Thorac Cardiovasc Surg 9(5):295–300
El-Gamel A, Sim E, Hasleton P, Hutchinson J, Yonan N, Egan J, Campbell C, Rahman A, Sheldon S, Deiraniya A, Hutchinson IV (1999) Transforming growth factor beta (TGF-beta) and obliterative bronchiolitis following pulmonary transplantation. J Heart Lung Transplant 18(9):828–837
Elssner A, Jaumann F, Dobmann S, Behr J, Schwaiblmair M, Reichenspurner H, Furst H, Briegel J, Vogelmeier C (2000) Elevated levels of interleukin-8 and transforming growth factor-beta in bronchoalveolar lavage fluid from patients with bronchiolitis obliterans syndrome: proinflammatory role of bronchial epithelial cells. Munich Lung Transplant Group. Transplantation 70(2):362–367
Takizawa H, Tanaka M, Takami K, Ohtoshi T, Ito K, Satoh M, Okada Y, Yamasawa F, Nakahara K, Umeda A (2001) Increased expression of transforming growth factor-beta1 in small airway epithelium from tobacco smokers and patients with chronic obstructive pulmonary disease (COPD). Am J Respir Crit Care Med 163(6):1476–1483
Soler N, Ewig S, Torres A, Filella X, Gonzalez J, Zaubet A (1999) Airway inflammation and bronchial microbial patterns in patients with stable chronic obstructive pulmonary disease. Eur Respir J 14(5):1015–1022
Ziegenhagen MW, Schrum S, Zissel G, Zipfel PF, Schlaak M, Muller-Quernheim J (1998) Increased expression of proinflammatory chemokines in bronchoalveolar lavage cells of patients with progressing idiopathic pulmonary fibrosis and sarcoidosis. J Investig Med 46(5):223–231
Bhowmik A, Seemungal TA, Sapsford RJ, Wedzicha JA (2000) Relation of sputum inflammatory markers to symptoms and lung function changes in COPD exacerbations. Thorax 55(2):114–120
Ear T, Fortin CF, Simard FA, McDonald PP. Constitutive association of TGF-beta-activated kinase 1 with the IkappaB kinase complex in the nucleus and cytoplasm of human neutrophils and its impact on downstream processes. J Immunol 184(7):3897–3906
Brandl M, Seidler B, Haller F, Adamski J, Schmid RM, Saur D, Schneider G. IKK(alpha) controls canonical TGF(ss)-SMAD signaling to regulate genes expressing SNAIL and SLUG during EMT in panc1 cells. J Cell Sci 123(Pt 24):4231–4239
Wajant H, Scheurich P. TNFR1-induced activation of the classical NF-kappaB pathway. FEBS J 278(6):862–876
Kuno K, Sukegawa K, Ishikawa Y, Orii T, Matsushima K (1994) Acid sphingomyelinase is not essential for the IL-1 and tumor necrosis factor receptor signaling pathway leading to NFkB activation. Int Immunol 6(8):1269–1272
Neil JR, Schiemann WP (2008) Altered TAB1:I kappaB kinase interaction promotes transforming growth factor beta-mediated nuclear factor-kappaB activation during breast cancer progression. Cancer Res 68(5):1462–1470
Huber MA, Azoitei N, Baumann B, Grunert S, Sommer A, Pehamberger H, Kraut N, Beug H, Wirth T (2004) NF-kappaB is essential for epithelial-mesenchymal transition and metastasis in a model of breast cancer progression. J Clin Invest 114(4):569–581
Chua HL, Bhat-Nakshatri P, Clare SE, Morimiya A, Badve S, Nakshatri H (2007) NF-kappaB represses E-cadherin expression and enhances epithelial to mesenchymal transition of mammary epithelial cells: potential involvement of ZEB-1 and ZEB-2. Oncogene 26(5):711–724
Lin CY, Lin CJ, Chen KH, Wu JC, Huang SH, Wang SM (2006) Macrophage activation increases the invasive properties of hepatoma cells by destabilization of the adherens junction. FEBS Lett 580(13):3042–3050
Luo Y, Zhou H, Krueger J, Kaplan C, Lee SH, Dolman C, Markowitz D, Wu W, Liu C, Reisfeld RA, Xiang R (2006) Targeting tumor-associated macrophages as a novel strategy against breast cancer. J Clin Invest 116(8):2132–2141
Acknowledgments
This work was supported by a research grant from the Medical Research Council UK (G0700861). LAB is supported by a Marie Curie fellowship award. AJF is supported by a GlaxoSmithKline clinical fellowship award. ADS is supported by a HEFCE Senior Lectureship. DAM is supported by the Wellcome Trust (WT086755MA).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Borthwick, L.A., Gardner, A., De Soyza, A. et al. Transforming Growth Factor-β1 (TGF-β1) Driven Epithelial to Mesenchymal Transition (EMT) is Accentuated by Tumour Necrosis Factor α (TNFα) via Crosstalk Between the SMAD and NF-κB Pathways. Cancer Microenvironment 5, 45–57 (2012). https://doi.org/10.1007/s12307-011-0080-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12307-011-0080-9