Abstract
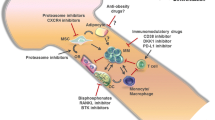
Myeloma bone disease (BD) not only impairs quality of life, but is also associated with impaired survival. Studies of the biology underlying BD support the notion that the increased osteoclastogenesis and suppressed osteoblastogenesis, is both a consequence and a necessity for tumour growth and clonal expansion. Survival and expansion of the myeloma clone is dependent on its interactions with bone elements, thus targeting these interactions should have antimyeloma activities. Indeed both experimental and clinical findings indicate that bone-targeted therapies not only improve BD, but also create an inhospitable environment for myeloma cell growth and survival, favouring improved clinical outcome. This review summarizes recent progress in our understandings of the biology of myeloma BD, highlighting the role of osteoclasts and osteoblasts in this process and how they can be targeted therapeutically. Unravelling the mechanisms underlying myeloma-bone interactions will facilitate the development of novel therapeutic agents to treat BD, which as a consequence are likely to improve the clinical outcome of myeloma patients.

Similar content being viewed by others
References
Melton LJ 3rd, Kyle RA, Achenbach SJ, Oberg AL, Rajkumar SV (2005) Fracture risk with multiple myeloma: a population-based study. J Bone Miner Res 20(3):487–493. doi:10.1359/JBMR.041131
Durie BG, Salmon SE (1975) A clinical staging system for multiple myeloma. Correlation of measured myeloma cell mass with presenting clinical features, response to treatment, and survival. Cancer 36(3):842–854
Jakob C, Sterz J, Liebisch P, Mieth M, Rademacher J, Goerke A, Heider U, Fleissner C, Kaiser M, von Metzler I, Muller C, Sezer O (2008) Incorporation of the bone marker carboxy-terminal telopeptide of type-1 collagen improves prognostic information of the International Staging System in newly diagnosed symptomatic multiple myeloma. Leukemia 22(9):1767–1772. doi:10.1038/leu.2008.159
Zhan F, Huang Y, Colla S, Stewart JP, Hanamura I, Gupta S, Epstein J, Yaccoby S, Sawyer J, Burington B, Anaissie E, Hollmig K, Pineda-Roman M, Tricot G, van Rhee F, Walker R, Zangari M, Crowley J, Barlogie B, Shaughnessy JD Jr (2006) The molecular classification of multiple myeloma. Blood 108(6):2020–2028. doi:10.1182/blood-2005-11-013458
Bataille R, Chappard D, Marcelli C, Dessauw P, Sany J, Baldet P, Alexandre C (1989) Mechanisms of bone destruction in multiple myeloma: the importance of an unbalanced process in determining the severity of lytic bone disease. J Clin Oncol 7(12):1909–1914
Fonseca R, Bailey RJ, Ahmann GJ, Rajkumar SV, Hoyer JD, Lust JA, Kyle RA, Gertz MA, Greipp PR, Dewald GW (2002) Genomic abnormalities in monoclonal gammopathy of undetermined significance. Blood 100(4):1417–1424
Mitsiades CS, McMillin DW, Klippel S, Hideshima T, Chauhan D, Richardson PG, Munshi NC, Anderson KC (2007) The role of the bone marrow microenvironment in the pathophysiology of myeloma and its significance in the development of more effective therapies. Hematol Oncol Clin North Am 21(6):1007–1034. doi:10.1016/j.hoc.2007.08.007, vii-viii
Hurt EM, Wiestner A, Rosenwald A, Shaffer AL, Campo E, Grogan T, Bergsagel PL, Kuehl WM, Staudt LM (2004) Overexpression of c-maf is a frequent oncogenic event in multiple myeloma that promotes proliferation and pathological interactions with bone marrow stroma. Cancer Cell 5(2):191–199
Robbiani DF, Chesi M, Bergsagel PL (2004) Bone lesions in molecular subtypes of multiple myeloma. N Engl J Med 351(2):197–198. doi:10.1056/NEJM200407083510223
Wu P, Walker BA, Boyd KD, Wardell CP, Johnson DC, Gregory WM, Davies FE, Brewer D, Morgan GJ (2010) Defining myeloma patients at high risk of developing bone disease while on bisphosphonate treatment blood (ASH Meeting Abstracts) 116:1
Bergsagel PL, Kuehl WM, Zhan F, Sawyer J, Barlogie B, Shaughnessy J Jr (2005) Cyclin D dysregulation: an early and unifying pathogenic event in multiple myeloma. Blood 106(1):296–303. doi:10.1182/blood-2005-01-0034
Bergsagel PL, Kuehl WM (2003) Critical roles for immunoglobulin translocations and cyclin D dysregulation in multiple myeloma. Immunol Rev 194:96–104
Hodge JM, Kirkland MA, Aitken CJ, Waugh CM, Myers DE, Lopez CM, Adams BE, Nicholson GC (2004) Osteoclastic potential of human CFU-GM: biphasic effect of GM-CSF. J Bone Miner Res 19(2):190–199. doi:10.1359/JBMR.0301232
Yaccoby S (2010) Advances in the understanding of myeloma bone disease and tumour growth. Br J Haematol 149(3):311–321. doi:10.1111/j.1365-2141.2010.08141.x
Yaccoby S, Wezeman MJ, Henderson A, Cottler-Fox M, Yi Q, Barlogie B, Epstein J (2004) Cancer and the microenvironment: myeloma-osteoclast interactions as a model. Cancer Res 64(6):2016–2023
Yaccoby S (2005) The phenotypic plasticity of myeloma plasma cells as expressed by dedifferentiation into an immature, resilient, and apoptosis-resistant phenotype. Clin Cancer Res 11(21):7599–7606. doi:10.1158/1078-0432.CCR-05-0523
Dezorella N, Pevsner-Fischer M, Deutsch V, Kay S, Baron S, Stern R, Tavor S, Nagler A, Naparstek E, Zipori D, Katz BZ (2009) Mesenchymal stromal cells revert multiple myeloma cells to less differentiated phenotype by the combined activities of adhesive interactions and interleukin-6. Exp Cell Res 315(11):1904–1913. doi:10.1016/j.yexcr.2009.03.016
Walker R, Barlogie B, Haessler J, Tricot G, Anaissie E, Shaughnessy JD Jr, Epstein J, van Hemert R, Erdem E, Hoering A, Crowley J, Ferris E, Hollmig K, van Rhee F, Zangari M, Pineda-Roman M, Mohiuddin A, Yaccoby S, Sawyer J, Angtuaco EJ (2007) Magnetic resonance imaging in multiple myeloma: diagnostic and clinical implications. J Clin Oncol 25(9):1121–1128. doi:10.1200/JCO.2006.08.5803
Bartel TB, Haessler J, Brown TL, Shaughnessy JD Jr, van Rhee F, Anaissie E, Alpe T, Angtuaco E, Walker R, Epstein J, Crowley J, Barlogie B (2009) F18-fluorodeoxyglucose positron emission tomography in the context of other imaging techniques and prognostic factors in multiple myeloma. Blood 114(10):2068–2076. doi:10.1182/blood-2009-03-213280
Zipori D (2010) The hemopoietic stem cell niche versus the microenvironment of the multiple myeloma-tumor initiating cell. Cancer Microenviron 3(1):15–28. doi:10.1007/s12307-009-0034-7
Podar K, Chauhan D, Anderson KC (2009) Bone marrow microenvironment and the identification of new targets for myeloma therapy. Leukemia 23(1):10–24. doi:10.1038/leu.2008.259
Glass DA 2nd, Bialek P, Ahn JD, Starbuck M, Patel MS, Clevers H, Taketo MM, Long F, McMahon AP, Lang RA, Karsenty G (2005) Canonical Wnt signaling in differentiated osteoblasts controls osteoclast differentiation. Dev Cell 8(5):751–764. doi:10.1016/j.devcel.2005.02.017
Qiang YW, Chen Y, Stephens O, Brown N, Chen B, Epstein J, Barlogie B, Shaughnessy JD Jr (2008) Myeloma-derived Dickkopf-1 disrupts Wnt-regulated osteoprotegerin and RANKL production by osteoblasts: a potential mechanism underlying osteolytic bone lesions in multiple myeloma. Blood 112(1):196–207. doi:10.1182/blood-2008-01-132134
Spencer GJ, Utting JC, Etheridge SL, Arnett TR, Genever PG (2006) Wnt signalling in osteoblasts regulates expression of the receptor activator of NFkappaB ligand and inhibits osteoclastogenesis in vitro. J Cell Sci 119(Pt 7):1283–1296. doi:10.1242/jcs.02883
Gunn WG, Conley A, Deininger L, Olson SD, Prockop DJ, Gregory CA (2006) A crosstalk between myeloma cells and marrow stromal cells stimulates production of DKK1 and interleukin-6: a potential role in the development of lytic bone disease and tumor progression in multiple myeloma. Stem Cells 24(4):986–991. doi:10.1634/stemcells.2005-0220
Yaccoby S, Wezeman MJ, Zangari M, Walker R, Cottler-Fox M, Gaddy D, Ling W, Saha R, Barlogie B, Tricot G, Epstein J (2006) Inhibitory effects of osteoblasts and increased bone formation on myeloma in novel culture systems and a myelomatous mouse model. Haematologica 91(2):192–199
Li X, Pennisi A, Yaccoby S (2008) Role of decorin in the antimyeloma effects of osteoblasts. Blood 112(1):159–168. doi:10.1182/blood-2007-11-124164
Goldoni S, Iozzo RV (2008) Tumor microenvironment: modulation by decorin and related molecules harboring leucine-rich tandem motifs. Int J Cancer 123(11):2473–2479. doi:10.1002/ijc.23930
Roodman GD (2004) Mechanisms of bone metastasis. N Engl J Med 350(16):1655–1664. doi:10.1056/NEJMra030831
Ehrlich LA, Roodman GD (2005) The role of immune cells and inflammatory cytokines in Paget’s disease and multiple myeloma. Immunol Rev 208:252–266. doi:10.1111/j.0105-2896.2005.00323.x
Wu X, Pan G, McKenna MA, Zayzafoon M, Xiong WC, McDonald JM (2005) RANKL regulates Fas expression and Fas-mediated apoptosis in osteoclasts. J Bone Miner Res 20(1):107–116. doi:10.1359/JBMR.041022
Giuliani N, Bataille R, Mancini C, Lazzaretti M, Barille S (2001) Myeloma cells induce imbalance in the osteoprotegerin/osteoprotegerin ligand system in the human bone marrow environment. Blood 98(13):3527–3533
Pearse RN, Sordillo EM, Yaccoby S, Wong BR, Liau DF, Colman N, Michaeli J, Epstein J, Choi Y (2001) Multiple myeloma disrupts the TRANCE/osteoprotegerin cytokine axis to trigger bone destruction and promote tumor progression. Proc Natl Acad Sci U S A 98(20):11581–11586. doi:10.1073/pnas.201394498
Terpos E, Szydlo R, Apperley JF, Hatjiharissi E, Politou M, Meletis J, Viniou N, Yataganas X, Goldman JM, Rahemtulla A (2003) Soluble receptor activator of nuclear factor kappaB ligand-osteoprotegerin ratio predicts survival in multiple myeloma: proposal for a novel prognostic index. Blood 102(3):1064–1069. doi:10.1182/blood-2003-02-0380
Seidel C, Hjertner O, Abildgaard N, Heickendorff L, Hjorth M, Westin J, Nielsen JL, Hjorth-Hansen H, Waage A, Sundan A, Borset M (2001) Serum osteoprotegerin levels are reduced in patients with multiple myeloma with lytic bone disease. Blood 98(7):2269–2271
Croucher PI, Shipman CM, Lippitt J, Perry M, Asosingh K, Hijzen A, Brabbs AC, van Beek EJ, Holen I, Skerry TM, Dunstan CR, Russell GR, Van Camp B, Vanderkerken K (2001) Osteoprotegerin inhibits the development of osteolytic bone disease in multiple myeloma. Blood 98(13):3534–3540
Han JH, Choi SJ, Kurihara N, Koide M, Oba Y, Roodman GD (2001) Macrophage inflammatory protein-1alpha is an osteoclastogenic factor in myeloma that is independent of receptor activator of nuclear factor kappaB ligand. Blood 97(11):3349–3353
Lentzsch S, Gries M, Janz M, Bargou R, Dorken B, Mapara MY (2003) Macrophage inflammatory protein 1-alpha (MIP-1 alpha) triggers migration and signaling cascades mediating survival and proliferation in multiple myeloma (MM) cells. Blood 101(9):3568–3573. doi:10.1182/blood-2002-08-2383
Terpos E, Politou M, Szydlo R, Goldman JM, Apperley JF, Rahemtulla A (2003) Serum levels of macrophage inflammatory protein-1 alpha (MIP-1alpha) correlate with the extent of bone disease and survival in patients with multiple myeloma. Br J Haematol 123(1):106–109
Vallet S, Raje N, Ishitsuka K, Hideshima T, Podar K, Chhetri S, Pozzi S, Breitkreutz I, Kiziltepe T, Yasui H, Ocio EM, Shiraishi N, Jin J, Okawa Y, Ikeda H, Mukherjee S, Vaghela N, Cirstea D, Ladetto M, Boccadoro M, Anderson KC (2007) MLN3897, a novel CCR1 inhibitor, impairs osteoclastogenesis and inhibits the interaction of multiple myeloma cells and osteoclasts. Blood 110(10):3744–3752. doi:10.1182/blood-2007-05-093294
Vallet S, Pozzi S, Patel K, Vaghela N, Fulciniti MT, Veiby P, Hideshima T, Santo L, Cirstea D, Scadden DT, Anderson KC, Raje N (2011) A novel role for CCL3 (MIP-1alpha) in myeloma-induced bone disease via osteocalcin downregulation and inhibition of osteoblast function. Leukemia. doi:10.1038/leu.2011.43
Choi SJ, Oba Y, Gazitt Y, Alsina M, Cruz J, Anderson J, Roodman GD (2001) Antisense inhibition of macrophage inflammatory protein 1-alpha blocks bone destruction in a model of myeloma bone disease. J Clin Invest 108(12):1833–1841. doi:10.1172/JCI13116
Oyajobi BO, Franchin G, Williams PJ, Pulkrabek D, Gupta A, Munoz S, Grubbs B, Zhao M, Chen D, Sherry B, Mundy GR (2003) Dual effects of macrophage inflammatory protein-1alpha on osteolysis and tumor burden in the murine 5TGM1 model of myeloma bone disease. Blood 102(1):311–319. doi:10.1182/blood-2002-12-3905
Bataille R, Chappard D, Alexandre C, Dessauw P, Sany J (1986) Importance of quantitative histology of bone changes in monoclonal gammopathy. Br J Cancer 53(6):805–810
Bataille R, Delmas PD, Chappard D, Sany J (1990) Abnormal serum bone Gla protein levels in multiple myeloma. Crucial role of bone formation and prognostic implications. Cancer 66(1):167–172
Evans CE, Galasko CS, Ward C (1989) Does myeloma secrete an osteoblast inhibiting factor? J Bone Joint Surg Br 71(2):288–290
Bataille R, Chappard D, Marcelli C, Dessauw P, Baldet P, Sany J, Alexandre C (1991) Recruitment of new osteoblasts and osteoclasts is the earliest critical event in the pathogenesis of human multiple myeloma. J Clin Invest 88(1):62–66. doi:10.1172/JCI115305
Bataille R, Chappard D, Marcelli C, Rossi JF, Dessauw P, Baldet P, Sany J, Alexandre C (1990) Osteoblast stimulation in multiple myeloma lacking lytic bone lesions. Br J Haematol 76(4):484–487
Atkins GJ, Kostakis P, Pan B, Farrugia A, Gronthos S, Evdokiou A, Harrison K, Findlay DM, Zannettino AC (2003) RANKL expression is related to the differentiation state of human osteoblasts. J Bone Miner Res 18(6):1088–1098. doi:10.1359/jbmr.2003.18.6.1088
Westendorf JJ, Kahler RA, Schroeder TM (2004) Wnt signaling in osteoblasts and bone diseases. Gene 341:19–39. doi:10.1016/j.gene.2004.06.044
Bain G, Muller T, Wang X, Papkoff J (2003) Activated beta-catenin induces osteoblast differentiation of C3H10T1/2 cells and participates in BMP2 mediated signal transduction. Biochem Biophys Res Commun 301(1):84–91
Tian E, Zhan F, Walker R, Rasmussen E, Ma Y, Barlogie B, Shaughnessy JD Jr (2003) The role of the Wnt-signaling antagonist DKK1 in the development of osteolytic lesions in multiple myeloma. N Engl J Med 349(26):2483–2494. doi:10.1056/NEJMoa030847
Giuliani N, Morandi F, Tagliaferri S, Lazzaretti M, Donofrio G, Bonomini S, Sala R, Mangoni M, Rizzoli V (2007) Production of Wnt inhibitors by myeloma cells: potential effects on canonical Wnt pathway in the bone microenvironment. Cancer Res 67(16):7665–7674. doi:10.1158/0008-5472.CAN-06-4666
Haaber J, Abildgaard N, Knudsen LM, Dahl IM, Lodahl M, Thomassen M, Kerndrup GB, Rasmussen T (2008) Myeloma cell expression of 10 candidate genes for osteolytic bone disease. Only overexpression of DKK1 correlates with clinical bone involvement at diagnosis. Br J Haematol 140(1):25–35. doi:10.1111/j.1365-2141.2007.06871.x
Politou MC, Heath DJ, Rahemtulla A, Szydlo R, Anagnostopoulos A, Dimopoulos MA, Croucher PI, Terpos E (2006) Serum concentrations of Dickkopf-1 protein are increased in patients with multiple myeloma and reduced after autologous stem cell transplantation. Int J Cancer 119(7):1728–1731. doi:10.1002/ijc.22033
Kaiser M, Mieth M, Liebisch P, Oberlander R, Rademacher J, Jakob C, Kleeberg L, Fleissner C, Braendle E, Peters M, Stover D, Sezer O, Heider U (2008) Serum concentrations of DKK-1 correlate with the extent of bone disease in patients with multiple myeloma. Eur J Haematol 80(6):490–494. doi:10.1111/j.1600-0609.2008.01065.x
Yaccoby S, Ling W, Zhan F, Walker R, Barlogie B, Shaughnessy JD Jr (2007) Antibody-based inhibition of DKK1 suppresses tumor-induced bone resorption and multiple myeloma growth in vivo. Blood 109(5):2106–2111. doi:10.1182/blood-2006-09-047712
Davies FE, Dring AM, Li C, Rawstron AC, Shammas MA, O’Connor SM, Fenton JA, Hideshima T, Chauhan D, Tai IT, Robinson E, Auclair D, Rees K, Gonzalez D, Ashcroft AJ, Dasgupta R, Mitsiades C, Mitsiades N, Chen LB, Wong WH, Munshi NC, Morgan GJ, Anderson KC (2003) Insights into the multistep transformation of MGUS to myeloma using microarray expression analysis. Blood 102(13):4504–4511. doi:10.1182/blood-2003-01-0016
De Vos J, Couderc G, Tarte K, Jourdan M, Requirand G, Delteil MC, Rossi JF, Mechti N, Klein B (2001) Identifying intercellular signaling genes expressed in malignant plasma cells by using complementary DNA arrays. Blood 98(3):771–780
Oshima T, Abe M, Asano J, Hara T, Kitazoe K, Sekimoto E, Tanaka Y, Shibata H, Hashimoto T, Ozaki S, Kido S, Inoue D, Matsumoto T (2005) Myeloma cells suppress bone formation by secreting a soluble Wnt inhibitor, sFRP-2. Blood 106(9):3160–3165. doi:10.1182/blood-2004-12-4940
Giuliani N, Colla S, Morandi F, Lazzaretti M, Sala R, Bonomini S, Grano M, Colucci S, Svaldi M, Rizzoli V (2005) Myeloma cells block RUNX2/CBFA1 activity in human bone marrow osteoblast progenitors and inhibit osteoblast formation and differentiation. Blood 106(7):2472–2483. doi:10.1182/blood-2004-12-4986
Li X, Ominsky MS, Warmington KS, Morony S, Gong J, Cao J, Gao Y, Shalhoub V, Tipton B, Haldankar R, Chen Q, Winters A, Boone T, Geng Z, Niu QT, Ke HZ, Kostenuik PJ, Simonet WS, Lacey DL, Paszty C (2009) Sclerostin antibody treatment increases bone formation, bone mass, and bone strength in a rat model of postmenopausal osteoporosis. J Bone Miner Res 24(4):578–588. doi:10.1359/jbmr.081206
Winkler DG, Sutherland MK, Geoghegan JC, Yu C, Hayes T, Skonier JE, Shpektor D, Jonas M, Kovacevich BR, Staehling-Hampton K, Appleby M, Brunkow ME, Latham JA (2003) Osteocyte control of bone formation via sclerostin, a novel BMP antagonist. EMBO J 22(23):6267–6276. doi:10.1093/emboj/cdg599
Terpos E, Christoulas D, Katodritou E, Bratengeier C, Lindner B, Harmelin S, Hawa G, Boutsikas G, Migkou M, Gavriatopoulou M, Michalis E, Pouli A, Kastritis E, Zervas K, Dimopoulos MA (2009) High serum sclerostin correlates with advanced stage, increased bone resporption, reduced osteoblast function, and poor survival in newly-diagnosed patients with multiple myeloma. Blood 114:1 (ASH Meeting Abstracts)
Colucci S, Brunetti G, Oranger A, Mori G, Sardone F, Liso V, Curci P, Miccolis RM, Rinaldi E, Specchia G, Passeri G, Zallone A, Rizzi R, Grano M (2010) Myeloma cells induce osteoblast suppression through sclerostin secretion blood (ASH Meeting Abstracts) 116:1
Krishnan V, Bryant HU, Macdougald OA (2006) Regulation of bone mass by Wnt signaling. J Clin Invest 116(5):1202–1209. doi:10.1172/JCI28551
Derksen PW, Tjin E, Meijer HP, Klok MD, MacGillavry HD, van Oers MH, Lokhorst HM, Bloem AC, Clevers H, Nusse R, van der Neut R, Spaargaren M, Pals ST (2004) Illegitimate WNT signaling promotes proliferation of multiple myeloma cells. Proc Natl Acad Sci U S A 101(16):6122–6127. doi:10.1073/pnas.0305855101
Edwards CM, Edwards JR, Lwin ST, Esparza J, Oyajobi BO, McCluskey B, Munoz S, Grubbs B, Mundy GR (2008) Increasing Wnt signaling in the bone marrow microenvironment inhibits the development of myeloma bone disease and reduces tumor burden in bone in vivo. Blood 111(5):2833–2842. doi:10.1182/blood-2007-03-077685
Zangari M, Esseltine D, Lee CK, Barlogie B, Elice F, Burns MJ, Kang SH, Yaccoby S, Najarian K, Richardson P, Sonneveld P, Tricot G (2005) Response to bortezomib is associated to osteoblastic activation in patients with multiple myeloma. Br J Haematol 131(1):71–73. doi:10.1111/j.1365-2141.2005.05733.x
Anderson G, Gries M, Kurihara N, Honjo T, Anderson J, Donnenberg V, Donnenberg A, Ghobrial I, Mapara MY, Stirling D, Roodman D, Lentzsch S (2006) Thalidomide derivative CC-4047 inhibits osteoclast formation by down-regulation of PU.1. Blood 107(8):3098–3105. doi:10.1182/blood-2005-08-3450
Terpos E, Dimopoulos MA, Sezer O (2007) The effect of novel anti-myeloma agents on bone metabolism of patients with multiple myeloma. Leukemia 21(9):1875–1884. doi:10.1038/sj.leu.2404843
Breitkreutz I, Raab MS, Vallet S, Hideshima T, Raje N, Mitsiades C, Chauhan D, Okawa Y, Munshi NC, Richardson PG, Anderson KC (2008) Lenalidomide inhibits osteoclastogenesis, survival factors and bone-remodeling markers in multiple myeloma. Leukemia 22(10):1925–1932. doi:10.1038/leu.2008.174
Kyle RA, Yee GC, Somerfield MR, Flynn PJ, Halabi S, Jagannath S, Orlowski RZ, Roodman DG, Twilde P, Anderson K (2007) American Society of Clinical Oncology 2007 clinical practice guideline update on the role of bisphosphonates in multiple myeloma. J Clin Oncol 25(17):2464–2472. doi:10.1200/JCO.2007.12.1269
Morgan GJ (2011) Can bisphosphonates improve outcomes in patients with newly diagnosed multiple myeloma? Crit Rev Oncol Hematol 77(Suppl 1):S24–S30. doi:10.1016/S1040-8428(11)70005-1
Aviles A, Nambo MJ, Neri N, Castaneda C, Cleto S, Huerta-Guzman J (2007) Antitumor effect of zoledronic acid in previously untreated patients with multiple myeloma. Med Oncol 24(2):227–230
McCloskey EV, Dunn JA, Kanis JA, MacLennan IC, Drayson MT (2001) Long-term follow-up of a prospective, double-blind, placebo-controlled randomized trial of clodronate in multiple myeloma. Br J Haematol 113(4):1035–1043
Berenson JR, Lichtenstein A, Porter L, Dimopoulos MA, Bordoni R, George S, Lipton A, Keller A, Ballester O, Kovacs M, Blacklock H, Bell R, Simeone JF, Reitsma DJ, Heffernan M, Seaman J, Knight RD (1998) Long-term pamidronate treatment of advanced multiple myeloma patients reduces skeletal events. Myeloma Aredia Study Group. J Clin Oncol 16(2):593–602
Morgan GJ, Davies FE, Gregory WM, Cocks K, Bell SE, Szubert AJ, Navarro-Coy N, Drayson MT, Owen RG, Feyler S, Ashcroft AJ, Ross F, Byrne J, Roddie H, Rudin C, Cook G, Jackson GH, Child JA (2010) First-line treatment with zoledronic acid as compared with clodronic acid in multiple myeloma (MRC Myeloma IX): a randomised controlled trial. Lancet 376(9757):1989–1999. doi:10.1016/S0140-6736(10)62051-X
Berenson J, Dimopoulos M, Chen YM (2006) Improved survival in patients with multiple myeloma and high BALP levels treated with zoledronic acid compared with pamidronate: univariate and multivariate models of hazard ratios. Blood (ASH Meeting Abstracts) 1
Rosen LS, Gordon D, Kaminski M, Howell A, Belch A, Mackey J, Apffelstaedt J, Hussein M, Coleman RE, Reitsma DJ, Seaman JJ, Chen BL, Ambros Y (2001) Zoledronic acid versus pamidronate in the treatment of skeletal metastases in patients with breast cancer or osteolytic lesions of multiple myeloma: a phase III, double-blind, comparative trial. Cancer J 7(5):377–387
Baulch-Brown C, Molloy TJ, Yeh SL, Ma D, Spencer A (2007) Inhibitors of the mevalonate pathway as potential therapeutic agents in multiple myeloma. Leuk Res 31(3):341–352. doi:10.1016/j.leukres.2006.07.018
Shipman CM, Rogers MJ, Apperley JF, Russell RG, Croucher PI (1997) Bisphosphonates induce apoptosis in human myeloma cell lines: a novel anti-tumour activity. Br J Haematol 98(3):665–672
Tassone P, Forciniti S, Galea E, Morrone G, Turco MC, Martinelli V, Tagliaferri P, Venuta S (2000) Growth inhibition and synergistic induction of apoptosis by zoledronate and dexamethasone in human myeloma cell lines. Leukemia 14(5):841–844
Ural AU, Yilmaz MI, Avcu F, Pekel A, Zerman M, Nevruz O, Sengul A, Yalcin A (2003) The bisphosphonate zoledronic acid induces cytotoxicity in human myeloma cell lines with enhancing effects of dexamethasone and thalidomide. Int J Hematol 78(5):443–449
Corso A, Ferretti E, Lunghi M, Zappasodi P, Mangiacavalli S, De Amici M, Rusconi C, Varettoni M, Lazzarino M (2005) Zoledronic acid down-regulates adhesion molecules of bone marrow stromal cells in multiple myeloma: a possible mechanism for its antitumor effect. Cancer 104(1):118–125. doi:10.1002/cncr.21104
Zwolak P, Manivel JC, Jasinski P, Kirstein MN, Dudek AZ, Fisher J, Cheng EY (2010) Cytotoxic effect of zoledronic acid-loaded bone cement on giant cell tumor, multiple myeloma, and renal cell carcinoma cell lines. J Bone Joint Surg Am 92(1):162–168. doi:10.2106/JBJS.H.01679
Schmidmaier R, Simsek M, Baumann P, Emmerich B, Meinhardt G (2006) Synergistic antimyeloma effects of zoledronate and simvastatin. Anticancer Drugs 17(6):621–629. doi:10.1097/01.cad.0000215058.85813.02
Moschetta M, Di Pietro G, Ria R, Gnoni A, Mangialardi G, Guarini A, Ditonno P, Musto P, D’Auria F, Ricciardi MR, Dammacco F, Ribatti D, Vacca A (2010) Bortezomib and zoledronic acid on angiogenic and vasculogenic activities of bone marrow macrophages in patients with multiple myeloma. Eur J Cancer 46(2):420–429. doi:10.1016/j.ejca.2009.10.019
Guenther A, Gordon S, Tiemann M, Burger R, Bakker F, Green JR, Baum W, Roelofs AJ, Rogers MJ, Gramatzki M (2010) The bisphosphonate zoledronic acid has antimyeloma activity in vivo by inhibition of protein prenylation. Int J Cancer 126(1):239–246. doi:10.1002/ijc.24758
Lipton A, Cook RJ, Coleman RE, Smith MR, Major P, Terpos E, Berenson JR (2007) Clinical utility of biochemical markers of bone metabolism for improving the management of patients with advanced multiple myeloma. Clin Lymphoma Myeloma 7(5):346–353
Raje N, Roodman GD (2011) Advances in the biology and treatment of bone disease in multiple myeloma. Clin Cancer Res 17(6):1278–1286. doi:10.1158/1078-0432.CCR-10-1804
Fizazi K, Lipton A, Mariette X, Body JJ, Rahim Y, Gralow JR, Gao G, Wu L, Sohn W, Jun S (2009) Randomized phase II trial of denosumab in patients with bone metastases from prostate cancer, breast cancer, or other neoplasms after intravenous bisphosphonates. J Clin Oncol 27(10):1564–1571. doi:10.1200/JCO.2008.19.2146
Vij R, Horvath N, Spencer A, Taylor K, Vadhan-Raj S, Vescio R, Smith J, Qian Y, Yeh H, Jun S (2009) An open-label, phase 2 trial of denosumab in the treatment of relapsed or plateau-phase multiple myeloma. Am J Hematol 84(10):650–656. doi:10.1002/ajh.21509
Henry DH, Costa L, Goldwasser F, Hirsh V, Hungria V, Prausova J, Scagliotti GV, Sleeboom H, Spencer A, Vadhan-Raj S, von Moos R, Willenbacher W, Woll PJ, Wang J, Jiang Q, Jun S, Dansey R, Yeh H (2011) Randomized, double-blind study of denosumab versus zoledronic Acid in the treatment of bone metastases in patients with advanced cancer (excluding breast and prostate cancer) or multiple myeloma. J Clin Oncol 29(9):1125–1132. doi:10.1200/JCO.2010.31.3304
Abe M, Hiura K, Wilde J, Shioyasono A, Moriyama K, Hashimoto T, Kido S, Oshima T, Shibata H, Ozaki S, Inoue D, Matsumoto T (2004) Osteoclasts enhance myeloma cell growth and survival via cell-cell contact: a vicious cycle between bone destruction and myeloma expansion. Blood 104(8):2484–2491. doi:10.1182/blood-2003-11-3839
Tai YT, Li XF, Breitkreutz I, Song W, Neri P, Catley L, Podar K, Hideshima T, Chauhan D, Raje N, Schlossman R, Richardson P, Munshi NC, Anderson KC (2006) Role of B-cell-activating factor in adhesion and growth of human multiple myeloma cells in the bone marrow microenvironment. Cancer Res 66(13):6675–6682. doi:10.1158/0008-5472.CAN-06-0190
Neri P, Kumar S, Fulciniti MT, Vallet S, Chhetri S, Mukherjee S, Tai Y, Chauhan D, Tassone P, Venuta S, Munshi NC, Hideshima T, Anderson KC, Raje N (2007) Neutralizing B-cell activating factor antibody improves survival and inhibits osteoclastogenesis in a severe combined immunodeficient human multiple myeloma model. Clin Cancer Res 13(19):5903–5909. doi:10.1158/1078-0432.CCR-07-0753
www.clinicaltrials.gov.
Shaugnessy J, Zhan F, Kordsmeier B, Randolph C, McCastlain K, Barlogie B (2002) Gene expression profiling (GEP) after short term in-vivo treatment identifies potential mechanisms of action of current drugs used to treat multiple myeloma. Blood 100:1 (ASH Meeting Abstracts)
Zavrski I, Krebbel H, Wildemann B, Heider U, Kaiser M, Possinger K, Sezer O (2005) Proteasome inhibitors abrogate osteoclast differentiation and osteoclast function. Biochem Biophys Res Commun 333(1):200–205. doi:10.1016/j.bbrc.2005.05.098
von Metzler I, Krebbel H, Hecht M, Manz RA, Fleissner C, Mieth M, Kaiser M, Jakob C, Sterz J, Kleeberg L, Heider U, Sezer O (2007) Bortezomib inhibits human osteoclastogenesis. Leukemia 21(9):2025–2034. doi:10.1038/sj.leu.2404806
Mukherjee S, Raje N, Schoonmaker JA, Liu JC, Hideshima T, Wein MN, Jones DC, Vallet S, Bouxsein ML, Pozzi S, Chhetri S, Seo YD, Aronson JP, Patel C, Fulciniti M, Purton LE, Glimcher LH, Lian JB, Stein G, Anderson KC, Scadden DT (2008) Pharmacologic targeting of a stem/progenitor population in vivo is associated with enhanced bone regeneration in mice. J Clin Invest 118(2):491–504. doi:10.1172/JCI33102
Giuliani N, Morandi F, Tagliaferri S, Lazzaretti M, Bonomini S, Crugnola M, Mancini C, Martella E, Ferrari L, Tabilio A, Rizzoli V (2007) The proteasome inhibitor bortezomib affects osteoblast differentiation in vitro and in vivo in multiple myeloma patients. Blood 110(1):334–338. doi:10.1182/blood-2006-11-059188
De Matteo M, Brunetti AE, Maiorano E, Cafforio P, Dammacco F, Silvestris F (2010) Constitutive down-regulation of Osterix in osteoblasts from myeloma patients: in vitro effect of Bortezomib and Lenalidomide. Leuk Res 34(2):243–249. doi:10.1016/j.leukres.2009.07.017
Terpos E, Heath DJ, Rahemtulla A, Zervas K, Chantry A, Anagnostopoulos A, Pouli A, Katodritou E, Verrou E, Vervessou EC, Dimopoulos MA, Croucher PI (2006) Bortezomib reduces serum dickkopf-1 and receptor activator of nuclear factor-kappaB ligand concentrations and normalises indices of bone remodelling in patients with relapsed multiple myeloma. Br J Haematol 135(5):688–692. doi:10.1111/j.1365-2141.2006.06356.x
Shimazaki C, Uchida R, Nakano S, Namura K, Fuchida SI, Okano A, Okamoto M, Inaba T (2005) High serum bone-specific alkaline phosphatase level after bortezomib-combined therapy in refractory multiple myeloma: possible role of bortezomib on osteoblast differentiation. Leukemia 19(6):1102–1103. doi:10.1038/sj.leu.2403758
Heider U, Kaiser M, Muller C, Jakob C, Zavrski I, Schulz CO, Fleissner C, Hecht M, Sezer O (2006) Bortezomib increases osteoblast activity in myeloma patients irrespective of response to treatment. Eur J Haematol 77(3):233–238. doi:10.1111/j.1600-0609.2006.00692.x
Boissy P, Andersen TL, Lund T, Kupisiewicz K, Plesner T, Delaisse JM (2008) Pulse treatment with the proteasome inhibitor bortezomib inhibits osteoclast resorptive activity in clinically relevant conditions. Leuk Res 32(11):1661–1668. doi:10.1016/j.leukres.2008.02.019
Uy GL, Trivedi R, Peles S, Fisher NM, Zhang QJ, Tomasson MH, DiPersio JF, Vij R (2007) Bortezomib inhibits osteoclast activity in patients with multiple myeloma. Clin Lymphoma Myeloma 7(9):587–589
Pennisi A, Li X, Ling W, Khan S, Zangari M, Yaccoby S (2009) The proteasome inhibitor, bortezomib suppresses primary myeloma and stimulates bone formation in myelomatous and nonmyelomatous bones in vivo. Am J Hematol 84(1):6–14. doi:10.1002/ajh.21310
Delforge M, Terpos E, Richardson PG, Shpilberg O, Khuageva NK, Schlag R, Dimopoulos MA, Kropff M, Spicka I, Petrucci MT, Samoilova OS, Mateos MV, Magen-Nativ H, Goldschmidt H, Esseltine DL, Ricci DS, Liu K, Deraedt W, Cakana A, van de Velde H, San Miguel JF (2011) Fewer bone disease events, improvement in bone remodeling, and evidence of bone healing with bortezomib plus melphalan-prednisone vs. melphalan-prednisone in the phase III VISTA trial in multiple myeloma. Eur J Haematol. doi:10.1111/j.1600-0609.2011.01599.x
Kuhns MA, Kalaycio M, Reu FJ, Maciejewski JP, Cheriyath V (2010) GSK-3β inhibitors in overcoming chemoresistance in multiple myeloma. Blood 116:1 [ASH abstract]
Gunn WG, Krause U, Lee N, Gregory CA (2011) Pharmaceutical inhibition of glycogen synthetase kinase-3beta reduces multiple myeloma-induced bone disease in a novel murine plasmacytoma xenograft model. Blood 117(5):1641–1651. doi:10.1182/blood-2010-09-308171
Kulkarni NH, Onyia JE, Zeng Q, Tian X, Liu M, Halladay DL, Frolik CA, Engler T, Wei T, Kriauciunas A, Martin TJ, Sato M, Bryant HU, Ma YL (2006) Orally bioavailable GSK-3alpha/beta dual inhibitor increases markers of cellular differentiation in vitro and bone mass in vivo. J Bone Miner Res 21(6):910–920. doi:10.1359/jbmr.060316
Kulkarni NH, Wei T, Kumar A, Dow ER, Stewart TR, Shou J, N’Cho M, Sterchi DL, Gitter BD, Higgs RE, Halladay DL, Engler TA, Martin TJ, Bryant HU, Ma YL, Onyia JE (2007) Changes in osteoblast, chondrocyte, and adipocyte lineages mediate the bone anabolic actions of PTH and small molecule GSK-3 inhibitor. J Cell Biochem 102(6):1504–1518. doi:10.1002/jcb.21374
Fulciniti M, Tassone P, Hideshima T, Vallet S, Nanjappa P, Ettenberg SA, Shen Z, Patel N, Tai YT, Chauhan D, Mitsiades C, Prabhala R, Raje N, Anderson KC, Stover DR, Munshi NC (2009) Anti-DKK1 mAb (BHQ880) as a potential therapeutic agent for multiple myeloma. Blood 114(2):371–379. doi:10.1182/blood-2008-11-191577
Vallet S, Mukherjee S, Vaghela N, Hideshima T, Fulciniti M, Pozzi S, Santo L, Cirstea D, Patel K, Sohani AR, Guimaraes A, Xie W, Chauhan D, Schoonmaker JA, Attar E, Churchill M, Weller E, Munshi N, Seehra JS, Weissleder R, Anderson KC, Scadden DT, Raje N (2010) Activin A promotes multiple myeloma-induced osteolysis and is a promising target for myeloma bone disease. Proc Natl Acad Sci U S A 107(11):5124–5129. doi:10.1073/pnas.0911929107
Chantry A, Heath D, Coulton L, Gallagher O, Evans H, Seehra J, Vanderkerken KICP (2007) A soluble activin type II receptor prevents myeloma bone disease. Haematologica 92(suppl2):1 (Abstract)
Lotinun S, Pearsall RS, Davies MV, Marvell TH, Monnell TE, Ucran J, Fajardo RJ, Kumar R, Underwood KW, Seehra J, Bouxsein ML, Baron R (2010) A soluble activin receptor Type IIA fusion protein (ACE-011) increases bone mass via a dual anabolic-antiresorptive effect in Cynomolgus monkeys. Bone 46(4):1082–1088. doi:10.1016/j.bone.2010.01.370
Abdulkadyrov KM, Salogub GN, Khuazheva NK, Woolf R, Haltom E, Borgstein NG, Knight R, Renshaw G, Yang Y, Sherman ML (2009) ACE-011, a soluble activin receptor type Iia IgG-Fc fusion protein, increases hemoglobin (Hb) and improves bone lesions in multiple myeloma patients receiving myelosuppressive chemotherapy: preliminary analysis. Blood 114:1 (ASH Meeting Abstracts)
Acknowledgements
Thanks to these organizations who have provided funding support to this article: Myeloma UK, Cancer Research UK, the Bud Flanagan Leukaemia Fund and the Biological Research Centre of the National Institute for Health Research at the Royal Marsden Hospital
Conflict of interest
The authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Wu, P., Morgan, G.J. Targeting Bone as a Therapy for Myeloma. Cancer Microenvironment 4, 299–311 (2011). https://doi.org/10.1007/s12307-011-0079-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12307-011-0079-2