Abstract
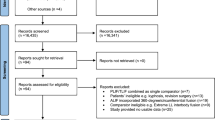
The aim of this meta-analysis and systematic review is to summarize and critically analyze the influence of surgery-related factors in lumbar interbody fusion for degenerative spine diseases. A systematic review of the literature was carried out with a primary search being performed on Medline through PubMed. The 2009 PRISMA flowchart and checklist were taken into account. Sixty-seven articles were included in the analysis: 48 studies were level IV of evidence, whereas 19 were level III. All interbody fusion techniques analyzed have proved to reach a good fusion rate. An overall mean fusion rate of 93% (95% CI 92–95%, p < 0.001) was estimated pooling the selected studies. The influence of sagittal parameters and cages features in fusion rate was not clear. Autograft is considered the gold standard material. The use of synthetic bone substitutes and biological factors alone or combined with bone graft have shown conflicting results. Low level of evidence studies and high heterogeneity (χ2 = 271.4, df = 72, p < 0.001; I2 = 73.5%, τ2 = 0.05) in data analysis could result in the risk of bias. Further high-quality studies would better clarify these results in the future.





Similar content being viewed by others
References
Chun D, Baker K, Wellington K et al (2015) Lumbar pseudarthrosis: a review of current diagnosis and treatment. Neurosurg Focus 39(4):E10
Basso M, Cavagnaro L, Zanirato A et al (2017) What is the clinical evidence on regenerative medicine in intervertebral disc degeneration? Musculoskelet Surg 101(2):93–104
DePalma AF, Rothman RH (1968) The nature of pseudarthrosis. Clin Orthop Relat Res 59:113–118
Carpenter CT, Dietz JW, Leung KY et al (1996) Repair of a pseudarthrosis of the lumbar spine. A functional outcome study. J Bone Joint Surg Am 78:712–720
Adogwa O, Verla T, Thompson P et al (2014) Affective disorders influence clinical outcomes after revision lumbar surgery in elderly patients with symptomatic adjacent-segment disease, recurrent stenosis, or pseudarthrosis: clinical article. J Neurosurg Spine 21:153–159
OCEBM Levels of Evidence Working Group*. The Oxford levels of evidence 2. Oxford Centre for Evidence-Based Medicine. http://www.cebm.net/index.aspx?o=5653. Accessed July 2019
Fujimori T, Le H, Schairer WW et al (2015) Does transforaminal lumbar interbody fusion have advantages over posterolateral lumbar fusion for degenerative spondylolisthesis? Glob Spine J 5:102–109
Høy K, Bünger C, Niederman B et al (2013) Transforaminal lumbar interbody fusion (TLIF) versus posterolateral instrumented fusion (PLF) in degenerative lumbar disorders: a randomized clinical trial with 2-year follow-up. Eur Spine J 22:2022–2029
Faundez AA, Schwender JD, Safriel Y et al (2009) Clinical and radiological outcome of anterior–posterior fusion versus transforaminal lumbar interbody fusion for symptomatic disc degeneration: a retrospective comparative study of 133 patients. Eur Spine J 18:203–211
Lee N, Kim K, Yi S et al (2017) Comparison of Outcomes of anterior, posterior, and transforaminal lumbar interbody fusion surgery at a single lumbar level with degenerative spinal disease. World Neurosurg 101:216–226
Malham GM, Parker R, Blecher C et al (2016) Choice of approach does not affect clinical and radiologic outcomes: a comparative cohort of patients having anterior lumbar interbody fusion and patients having lateral lumbar interbody fusion at 24 months. Glob Spine J 6:472–481
Rodgers WB, Gerber EJ, Patterson JR et al (2010) Fusion after minimally disruptive anterior lumbar interbody fusion. Analysis of extreme lateral interbody fusion by computed tomography. SAS J 4:63–66
Marchi L, Oliveira L, Amaral R, Castro C et al (2012) Lateral interbody fusion for treatment of discogenic low back pain: minimally invasive surgical techniques. Adv Orthop 2012:282068
Ozgur BM, Agarwal V, Nail E et al (2010) Two-year clinical and radiographic success of minimally invasive lateral transpsoas approach for the treatment of degenerative lumbar conditions. SAS J 4:41–46
Malham GM, Ellis NJ, Parker RM et al (2012) Clinical outcome and fusion rates after the first 30 extreme lateral interbody fusions. ScientificWorldJournal 2012:246989
Berjano P, Langella F, Damilano M et al (2015) Fusion rate following extreme lateral lumbar interbody fusion. Eur Spine J 24(Suppl 3):369–371
Formica M, Berjano P, Cavagnaro L et al (2014) Extreme lateral approach to the spine in degenerative and post traumatic lumbar diseases: selection process, results and complications. Eur Spine J 23(Suppl 6):684–692
Norotte G, Barrios C (2018) Clinical and radiological outcomes after stand-alone ALIF for single L5-S1 degenerative discopathy using a PEEK cage filled with hydroxyapatite nanoparticles without bone graft. Clin Neurol Neurosurg 168:24–29
Song D, Tang M, Li C et al (2018) Double-level isthmic spondylolisthesis treated with posterior lumbar interbody fusion with cage. Br J Neurosurg 23:1–5
Massie LW, Zakaria HM, Schultz LR et al (2018) Assessment of radiographic and clinical outcomes of an articulating expandable interbody cage in minimally invasive transforaminal lumbar interbody fusion for spondylolisthesis. Neurosurg Focus 44(1):E8
Lee DD, Kim JY et al (2017) A comparison of radiographic and clinical outcomes of anterior lumbar interbody fusion performed with either a cellular bone allograft containing multipotent adult progenitor cells or recombinant human bone morphogenetic protein-2. J Orthop Surg Res 12(1):126
Seo DK, Kim MJ, Roh SW et al (2017) Morphological analysis of interbody fusion following posterior lumbar interbody fusion with cages using computed tomography. Medicine (Baltimore) 96(34):e7816
Khan TR, Pearce KR, McAnany SJ et al (2018) Comparison of transforaminal lumbar interbody fusion outcomes in patients receiving rhBMP-2 versus autograft. Spine J 18(3):439–446
Lee CW, Yoon KJ, Ha SS et al (2017) Which approach is advantageous to preventing development of adjacent segment disease? Comparative analysis of 3 different lumbar interbody fusion techniques (ALIF, LLIF, and PLIF) in L4-5 spondylolisthesis. World Neurosurg 105:612–622
Mobbs RJ, Phan K, Assem Y et al (2016) Combination Ti/PEEK ALIF cage for anterior lumbar interbody fusion: early clinical and radiological results. J Clin Neurosci 34:94–99
Saville PA, Kadam AB, Smith HE et al (2016) Anterior hyperlordotic cages: early experience and radiographic results. J Neurosurg Spine 25(6):713–719
Witoon N, Tangviriyapaiboon T et al (2014) Clinical and radiological outcomes of segmental spinal fusion in transforaminal lumbar interbody fusion with spinous process tricortical autograft. Asian Spine J 8(2):170–176
Alimi M, Hofstetter CP, Cong GT et al (2014) Radiological and clinical outcomes following extreme lateral interbody fusion. J Neurosurg Spine 20(6):623–635
Lee YS, Kim YB, Park SW et al (2014) Comparison of transforaminal lumbar interbody fusion with direct lumbar interbody fusion: clinical and radiological results. J Korean Neurosurg Soc 56(6):469–474
Hironaka Y, Morimoto T, Motoyama Y et al (2013) Surgical management of minimally invasive anterior lumbar interbody fusion with stand-alone interbody cage for L4-5 degenerative disorders: clinical and radiographic findings. Neurol Med Chir (Tokyo) 53(12):861–869
Bin Abd Razak HR, Dhoke P, Tay KS et al (2017) Single-level minimally invasive transforaminal lumbar interbody fusion provides sustained improvements in clinical and radiological outcomes up to 5 years postoperatively in patients with neurogenic symptoms secondary to spondylolisthesis. Asian Spine J 11(2):204–212
Giorgi H, Prébet R, Delhaye M, French Society of Spine Surgery (SFCR) et al (2015) Minimally invasive posterior transforaminal lumbar interbody fusion: one-year postoperative morbidity, clinical and radiological results of a prospective multicenter study of 182 cases. Orthop Traumatol Surg Res 101(6):S241–S245
Asazuma T, Masuoka K, Motosuneya T et al (2005) Posterior lumbar interbody fusion using dense hydroxyapatite blocks and autogenous iliac bone: clinical and radiographic examinations. J Spinal Disord Tech 18(Suppl):S41–S47
Burkus JK, Dorchak JD, Sanders DL et al (2003) Radiographic assessment of interbody fusion using recombinant human bone morphogenetic protein type 2. Spine (Phila Pa 1976) 28(4):372–377
Cheung KM, Zhang YG, Lu DS et al (2003) Reduction of disc space distraction after anterior lumbar interbody fusion with autologous iliac crest graft. Spine (Phila Pa 1976) 28(13):1385–1389
Carter JD, Swearingen AB, Chaput CD et al (2009) Clinical and radiographic assessment of transforaminal lumbar interbody fusion using HEALOS collagen-hydroxyapatite sponge with autologous bone marrow aspirate. Spine J 9(6):434–438
Cutler AR, Siddiqui S, Mohan AL et al (2006) Comparison of polyetheretherketone cages with femoral cortical bone allograft as a single-piece interbody spacer in transforaminal lumbar interbody fusion. J Neurosurg Spine 5(6):534–539
El Masry MA, Badawy WS, Rajendran P et al (2004) Combined anterior interbody fusion and posterior pedicle screw fixation in patients with degenerative lumbar disc disease. Int Orthop 28(5):294–297
Fogel GR, Toohey JS, Neidre A et al (2007) Is one cage enough in posterior lumbar interbody fusion: a comparison of unilateral single cage interbody fusion to bilateral cages. J Spinal Disord Tech 20(1):60–65
Hackenberg L, Halm H, Bullmann V et al (2005) Transforaminal lumbar interbody fusion: a safe technique with satisfactory three to five year results. Eur Spine J 14(6):551–558
Haid RW Jr, Branch CL Jr, Alexander JT et al (2004) Posterior lumbar interbody fusion using recombinant human bone morphogenetic protein type 2 with cylindrical interbody cages. Spine J 4(5):527–538 (discussion 538-9)
Huang KF, Chen TY (2003) Clinical results of a single central interbody fusion cage and transpedicle screws fixation for recurrent herniated lumbar disc and low-grade spondylolisthesis. Chang Gung Med J 26(3):170–177
Ito Z, Imagama S, Kanemura T et al (2013) Bone union rate with autologous iliac bone versus local bone graft in posterior lumbar interbody fusion (PLIF): a multicenter study. Eur Spine J 22(5):1158–1163
Kai Y, Oyama M, Morooka M et al (2004) Posterior lumbar interbody fusion using local facet joint autograft and pedicle screw fixation. Spine (Phila Pa 1976) 29(1):41–46
Ito Z, Matsuyama Y, Sakai Y et al (2010) Bone union rate with autologous iliac bone versus local bone graft in posterior lumbar interbody fusion. Spine (Phila Pa 1976) 35(21):E1101–E1105
Kalb S, Perez-Orribo L, Kalani MY et al (2016) The influence of common medical conditions on the outcome of anterior lumbar interbody fusion. Clin Spine Surg 29(7):285–290
Kim H, Lee CK, Yeom JS et al (2012) The efficacy of porous hydroxyapatite bone chip as an extender of local bone graft in posterior lumbar interbody fusion. Eur Spine J 21(7):1324–1330
Kim JS, Jung B, Lee SH et al (2018) Instrumented minimally invasive spinal-transforaminal lumbar interbody fusion (MIS-TLIF); minimum 5-years follow-up with clinical and radiologic outcomes. J Spinal Disord Tech 31(6):E302–E309
Jenis LG, Banco RJ, Kwon B et al (2006) A prospective study of Autologous Growth Factors (AGF) in lumbar interbody fusion. Spine J 6(1):14–20
Kim JW, Park HC, Yoon SH et al (2007) A multi-center clinical study of posterior lumbar interbody fusion with the expandable stand-alone cage (Tyche(R) Cage) for degenerative lumbar spinal disorders. J Korean Neurosurg Soc. 42(4):251–257
Kim MC, Chung HT, Kim DJ, Kim SH, Jeon SH (2011) The clinical and radiological outcomes of minimally invasive transforaminal lumbar interbody single level fusion. Asian Spine J 5(2):111–116
Lee JH, Jeon DW, Lee SJ et al (2010) Fusion rates and subsidence of morselized local bone grafted in titanium cages in posterior lumbar interbody fusion using quantitative three-dimensional computed tomography scans. Spine (Phila Pa 1976) 35(15):1460–1465
Lee JH, Lee JH, Park JW et al (2011) Fusion rates of a morselized local bone graft in polyetheretherketone cages in posterior lumbar interbody fusion by quantitative analysis using consecutive three-dimensional computed tomography scans. Spine J 11(7):647–653
Madan SS, Boeree NR et al (2003) Comparison of instrumented anterior interbody fusion with instrumented circumferential lumbar fusion. Eur Spine J 12(6):567–575
Miura Y, Imagama S, Yoda M et al (2003) Is local bone viable as a source of bone graft in posterior lumbar interbody fusion? Spine (Phila Pa 1976) 28(20):2386–2389
Mofidi A, Sedhom M, O’Shea K et al (2005) Is high level of disability an indication for spinal fusion? Analysis of long-term outcome after posterior lumbar interbody fusion using carbon fiber cages. J Spinal Disord Tech 18(6):479–484
Nakashima H, Yukawa Y, Ito K et al (2011) Extension CT scan: its suitability for assessing fusion after posterior lumbar interbody fusion. Eur Spine J 20(9):1496–1502
Okuyama K, Kido T, Unoki E et al (2007) PLIF with a titanium cage and excised facet joint bone for degenerative spondylolisthesis in augmentation with a pedicle screw. J Spinal Disord Tech 220(1):53–59
Park JH, Roh SW (2011) Long-term clinical and radiological outcomes following stand-alone PLIF surgery using expandable cylindrical threaded cages in patients with degenerative lumbar spine disease. Acta Neurochir 153(7):1409–1416
Pavlov PW, Meijers H, van Limbeek J et al (2004) Good outcome and restoration of lordosis after anterior lumbar interbody fusion with additional posterior fixation. Spine (Phila Pa 1976) 29(17):1893–1899 (discussion 1900)
Potter BK, Freedman BA, Verwiebe EG et al (2005) Transforaminal lumbar interbody fusion: clinical and radiographic results and complications in 100 consecutive patients. J Spinal Disord Tech. 18(4):337–346
Rouben D, Casnellie M, Ferguson M (2011) Long-term durability of minimal invasive posterior transforaminal lumbar interbody fusion: a clinical and radiographic follow-up. J Spinal Disord Tech. 24(5):288–296
Santos ER, Goss DG, Morcom RK et al (2003) Radiologic assessment of interbody fusion using carbon fiber cages. Spine (Phila Pa 1976) 28(10):997–1001
Schiffman M, Brau SA, Henderson R et al (2003) Bilateral implantation of low-profile interbody fusion cages: subsidence, lordosis, and fusion analysis. Spine J 3(5):377–387
Thaler M, Lechner R, Gstöttner M et al (2013) The use of beta-tricalcium phosphate and bone marrow aspirate as a bone graft substitute in posterior lumbar interbody fusion. Eur Spine J 22(5):1173–1182
Thalgott JS, Klezl Z, Timlin M et al (2002) Anterior lumbar interbody fusion with processed sea coral (coralline hydroxyapatite) as part of a circumferential fusion. Spine (Phila Pa 1976) 27(24):E518–E525 (discussion E526-7)
Thalgott JS, Giuffre JM, Klezl Z et al (2002) Anterior lumbar interbody fusion with titanium mesh cages, coralline hydroxyapatite, and demineralized bone matrix as part of a circumferential fusion. Spine J 2(1):63–69
Rihn JA, Makda J, Hong J et al (2009) The use of RhBMP-2 in single-level transforaminal lumbar interbody fusion: a clinical and radiographic analysis. Eur Spine J 18(11):1629–1636
Xiao Y, Li F, Chen Q (2010) Transforaminal lumbar interbody fusion with one cage and excised local bone. Arch Orthop Trauma Surg 130(5):591–597
Zhang YF, Yang HL, Wang JW et al (2009) Two-year follow-up results after treatment of lumbar instability with titanium-coated fusion system. Orthop Surg 1(2):94–100
Xue H, Tu Y, Cai M (2012) Comparison of unilateral versus bilateral instrumented transforaminal lumbar interbody fusion in degenerative lumbar diseases. Spine J 12(3):209–215
Zairi F, Arikat A, Allaoui M et al (2013) Transforaminal lumbar interbody fusion: comparison between open and mini-open approaches with two years follow-up. J Neurol Surg A Cent Eur Neurosurg 74(3):131–135
Ould-Slimane M, Lenoir T, Dauzac C et al (2012) Influence of transforaminal lumbar interbody fusion procedures on spinal and pelvic parameters of sagittal balance. Eur Spine J 21(6):1200–1206
Okuyama K, Abe E, Suzuki T et al (2001) Influence of bone mineral density on pedicle screw fixation: a study of pedicle screw fixation augmenting posterior lumbar interbody fusion in elderly patients. Spine J1:402–407
Choudhri TF, Mummaneni PV, Dhall SS et al (2014) Guideline update for the performance of fusion procedures for degenerative disease of the lumbar spine. Part 4: radiographic assessment of fusion status. J Neurosurg Spine 21:23–30
Bach K, Ahmadian A, Deukmedjian A et al (2014) Minimally invasive surgical techniques in adult degenerative spinal deformity: a systematic review. Clin Orthop Relat Res 472(6):1749–1761
Uribe JS, Smith DA, Dakwar E et al (2012) Lordosis restoration after anterior longitudinal ligament release and placement of lateral hyperlordotic interbody cages during the minimally invasive lateral transpsoas approach: a radiographic study in cadavers. J Neurosurg Spine 17(5):476–485
Formica M, Zanirato A, Cavagnaro L et al (2017) Extreme lateral interbody fusion in spinal revision surgery: clinical results and complications. Eur Spine J 26(suppl 4):464–470
Formica M, Cavagnaro L, Basso M, Zanirato A, Felli L, Formica C (2015) Is it possible to preserve lumbar lordosis after hybrid stabilization? Preliminary results of a novel rigid–dynamic stabilization system in degenerative lumbar pathologies. Eur Spine J 24(Suppl 7):S849–S854
Formica M, Divano S, Cavagnaro L et al (2017) Lumbar total disc arthroplasty: outdated surgery or here to stay procedure? A systematic review of current literature. J Orthop Traumatol 18(3):197–215
Hsieh PC, Koski TR, O’Shaughnessy BA et al (2007) Anterior lumbar interbody fusion in comparison with transforaminal lumbar interbody fusion: implications for the restoration of foraminal height, local disc angle, lumbar lordosis, and sagittal balance. J Neurosurg Spine 7:379–386
Hsu WK, Nickoli MS, Wang JC et al (2012) Improving the clinical evidence of bone graft substitute technology in lumbar spine surgery. Global Spine J 2:239–248
Gologorsky Y, Skovrlj B, Steinberger J et al (2014) Increased incidence of pseudarthrosis after unilateral instrumented transforaminal lumbar interbody fusion in patients with lumbar spondylosis. J Neurosurg Spine 21:601–607
Marengo N, Ajello M, Pecoraro MF et al (2018) Cortical bone trajectory screws in posterior lumbar interbody fusion: minimally invasive surgery for maximal muscle sparing—a prospective comparative study with the traditional open technique. Biomed Res Int 2018:7424568
Ailon T, Hamilton K, Klineberg E et al (2018) Radiographic fusion grade does not impact health related quality of life in the absence of instrumentation failure for patients undergoing posterior instrumented fusion for adult spinal deformity. World Neurosurg 117:e1–e7
Andersen T, Christensen FB, Laursen M et al (2001) Smoking as a predictor of negative outcome in lumbar spinal fusion. Spine (Phila Pa 1976) 26:2623–2628
Macki M, Syeda S, Rajjoub KR et al (2017) The effect of smoking status on successful arthrodesis after lumbar instrumentation supplemented with rhBMP-2. World Neurosurg 97:459–464
Glassman SD, Alegre G, Carreon L et al (2003) Perioperative complications of lumbar instrumentation and fusion in patients with diabetes mellitus. Spine J 3:496–501
Inose H, Yamada T, Mulati M et al (2018) Bone turnover markers as a new predicting factor for nonunion after spinal fusion surgery. Spine (Phila Pa 1976) 43(1):E29–E34
Chin DK, Park JY, Yoon YS et al (2007) Prevalence of osteoporosis in patients requiring spine surgery: incidence and significance of osteoporosis in spine disease. Osteoporos Int 18(9):1219–1224
Okuyama K, Abe E, Suzuki T et al (2001) Influence of bone mineral density on pedicle screw fixation: a study of pedicle screw fixation augmenting posterior lumbar interbody fusion in elderly patients. Spine J 1:402–407
Chen F, Dai Z, Kang Y et al (2016) Effects of zoledronic acid on bone fusion in osteoporotic patients after lumbar fusion. Osteoporos Int 27(4):1469–1476
Ebata S, Takahashi J, Hasegawa T et al (2017) Role of weekly teriparatide administration in osseous union enhancement within six months after posterior or transforaminal lumbar interbody fusion for osteoporosis-associated lumbar degenerative disorders. J Bone Joint Surg Am 99:365–372
Funding
No sources of funding for this systematic review and other support.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that there is no conflict of interest.
Ethical approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Formica, M., Vallerga, D., Zanirato, A. et al. Fusion rate and influence of surgery-related factors in lumbar interbody arthrodesis for degenerative spine diseases: a meta-analysis and systematic review. Musculoskelet Surg 104, 1–15 (2020). https://doi.org/10.1007/s12306-019-00634-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12306-019-00634-x