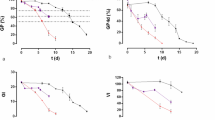
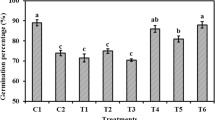
Abstract
Previous studies associated with seed potentiation support the critical role of metabolic readjustment in restricting the loss of seed vigor and viability of aged seeds. However, their exact role in the regulation of ‘oxidative windows’ of potentiated seeds is rarely studied and hence is the subject of the present investigation. Seed potentiation of two contrasting indigenous aromatic rice cultivars, differing in sensitivity towards redox attributes (Oryza sativa L., Cultivars Tulaipanji and Jamainadu), with standardized doses of hydrogen peroxide (20 mM), triadimefon (250 μM), herbal extract (1% aqueous extract of Lantana camara flower) and distilled water before accelerated aging (RH 92% and 41 °C for 24 h) found to have significant impact on redox regulation of aged seeds and improvement of germination phenotypes. The efficacy of integrated RBOH-ascorbate–glutathione/catalase pathway, redox status and other redox fingerprints in the metabolic landscape of potentiated-aged seeds vis-a-vis non-potentiated-aged seeds corroborate the impact of seed potentiation on the regulation of ‘oxidative window’ of experimental rice seeds. Gene expression analysis of central redox hub enzymes (Osrboh, OsAPx2, OsGRase, OsCatA) strongly substantiates the impact of seed potentiation on transcriptional regulation of genes for redox homeostasis in accelerated aged seeds. The novelty of the current effort is that it suggests a positive nexus between seed potentiation-induced redox regulation and hormonal homeostasis. The efficacy of seed potentiation on the redox regulation of experimental accelerated aged seeds is found to be cultivar-specific and comparatively better in the cultivar Tulaipanji as compared to the cultivar Jamainadu and in the order herbal extract, hydrogen peroxide, hydropriming and triadimefon.








Similar content being viewed by others
References
Adetunji AE, Adetunji TL, Varghese B, Sershen Pammenter NW (2021) Oxidative stress, aging and methods of seed invigoration: an overview and perspectives. Agronomy 11:2369. https://doi.org/10.3390/agronomy11122369
Arab L, Ehsanpour AA (2012) Improvement of some physiological responses of alfalfa (Medicago sativa L.) under in vitro salt stress using Triadimefon. Prog Biol Sci 3(1):31–40
Bailly C, El-Maarouf-Bouteau H, Corbineau F (2008) From intracellular signaling networks to cell death: the dual role of reactive oxygen species in seed physiology. C R Biol 331(10):806–814
Basra SMA, Iftikhar MN, Afzal I (2011) Potential of moringa (Moringa oleifera) leaf extract as priming agent for hybrid maize seeds. Int J Agric Biol 13(6):1006–1010
Bhattacharjee S (2008) Calcium-dependent signaling pathway in heat induced oxidative injury in Amaranthus lividus. Plant Biol 52(1):137–140
Bhattacharjee S, Dey N (2018) Redox metabolic and molecular parameters for screening drought tolerant indigenous aromatic rice cultivars. Physiol Mol Biol Plants 24(1):7–23
Buege JA, Aust SD (1978) Microsomal lipid peroxidation. Method Enzymol 52:302–310
Burch-Smith TM, Schiff M, Liu Y, Dinesh-Kumar SP (2006) Efficient virus induced gene silencing in Arabidopsis. Plant Physiol 142(1):21–27
Chaitanya KSK, Naithani SC (1994) Role of superoxide, lipid peroxidation and superoxide dismutase in membrane perturbation during loss of viability of Shorea robusta Gaertn F. New Phytol 126(4):623–627
Chang C, Yang M, Wen H, Chern J (2002) Estimation of total flavonoid content in propolis by two complementary colorimetric methods. Food Drug Anal 10:178–182
Chen K, Arora R (2013) Priming memory invokes seed stress tolerance. Environ Exp Bot 94:33–45
Chen X, Zhang R, Xing Y, Jiang B, Li B, Xu X et al (2021) The efficacy of different seed priming agents for promoting sorghum germination under salt stress. PLoS ONE 16(1):e0245505
Devasagayam TP, Boloor KK, Ramasarma T (2003) Methods for estimating lipid peroxidation: an analysis of merits and demerits. Indian J Biochem Biophys 40(5):300–308
Dey N, Bhattacharjee S (2020) Accumulation of phenolic compounds and osmolytes under dehydration stress and their implication in redox regulation: a biochemical basis for screening Indigenous Aromatic Rice Cultivars. Rice Sci 27(4):329–344
Dey A, Bhattacharjee S (2022) Temporal regulation of oxidative window and hormonal homeostasis are the key events regulating germination under salinity and oxidative stress. J Plant Growth Regul. https://doi.org/10.1007/s00344-022-10756-5a
Draganic I, Lekic S (2012) Seed priming with antioxidants improves sunflower seed germination and seedling growth under unfavorable germination conditions. Turk J Agric for 36(4):421–428
Ebone LA, Caverzan A, Chavarri G (2019) Physiologic alterations in orthodox seeds due to deterioration processes. Plant Physiol Biochem 145:34–42
Ella ES, Dionisio-Sese ML, Ismail AM (2011) Seed pre-treatment in rice reduces damage, enhances carbohydrate mobilization and improves emergence and seedling establishment under flooded conditions. AoB-Plants plr0007:1093–2000. https://doi.org/10.1093/aobpla/plr007
El-Maarouf-Bouteau H, Bailly C (2008) Oxidative signaling in seed germination and dormancy. Plant Signal Behav 3(3):175–182
Farooq M, Hussain M, Habib MM, Khan MS, Ahmad I, Farooq S et al (2020) Influence of seed priming techniques on grain yield and economic returns of bread wheat planted at different spacings. Crop past Sci 71(8):725–738. https://doi.org/10.1071/CP20065
Forti C, Ottobrino V, Bassolino L, Toppino L, Rotino GL, Pagano A, Macovei A, Balestrazzi A (2020) Molecular dynamics of pre-germinative metabolism in primed eggplant (Solanum melongena L.) seeds. Hortic Res 7(1):87
Foyer CH, Noctor G (2016) Stress-triggered redox signalling: what’s in pROSpect? Plant Cell Environ 39(5):951–964
Golldack D, Li C, Mohan H, Probst N (2013) Gibberellins and abscisic acid signal crosstalk: living and developing under unfavorable conditions. Plant Cell Rep 32(7):1007–1016. https://doi.org/10.1007/s00299-013-1409-2
Gong D, He F, Liu J, Zhang C, Wang Y, Tian S, Sun C, Zhang X (2022) Understanding of hormonal regulation in rice seed germination. Life 12(7):1021
He L, Gao Z, Li R (2009) Pretreatment of seed with H2O2 enhances drought tolerance of wheat (Triticum aestivum L.) seedlings. Afr J Biotechnol 08(22):6151–6157. https://doi.org/10.5897/AJB09.490
Heath RL, Packer L (1968) Photoperoxidation in isolated chloroplasts. I. Kinetics and stoichiometry of fatty acid peroxidation. Arch Biochem Biophys 125(1):189–198
Hossain MA, Bhattacharjee S, Armin S-M, Qian P, Xin W, Li H-Y, Burritt DJ, Fujita M, Tran L-SP (2015) Hydrogen peroxide priming modulates abiotic oxidative stress tolerance: insights from ROS detoxification and scavenging. Front Plant Sci 6:420. https://doi.org/10.3389/fpls.2015.00420
Illangakoon TK, Ella ES, Ismail AM, Marambe B, Keerthisena RS, Bentota AP, Kulatunge S (2016) Impact of variety and seed priming on anaerobic germination-tolerance of rice (Oryza sativa L.) varieties in Sri Lanka. Trop Agric Res 28(1):26–37
Jaleel C, Gopi R (2007) Responses of antioxidant defense system of Catharanthus roseus (L.) G. Don. To paclobutrazol treatment under salinity. Acta Physiol Plant 29(3):205–209
Jiang M, Zhang J (2001) Effect of abscisic acid on active oxygen species, antioxidative defense system and oxidative damage in leaves of maize seedlings. Plant Cell Physiol 42(11):1265–1273
Jisha KC, Vijayakumari K, Puthur JT (2013) Seed priming for abiotic stress tolerance: an overview. Acta Physiol Plant 35(5):1381–1396
Kaur N, Sharma I, Kirat K, Pati PK (2016) Detection of reactive oxygen species in Oryza sativa L. (Rice). Bio-Protoc 6(24):2061. https://doi.org/10.21769/bioprotoc.2061
Kibinza S, Bazin J, Bailly C, Farrant JM, Corbineau F, El-Maarouf-Bouteau H (2011) Catalase is a key enzyme in seed recovery from aging during priming. Plant Sci 181(3):309–315
Kumar P, Aeron A, Shaw N, Singh A, Bajpai VK, Pant S et al (2020) Seed bio-priming with tri-species consortia of phosphate solubilizing rhizobacteria (PSR) and its effect on plant growth promotion. Heliyon 6(12):e05701. https://doi.org/10.1016/j.heliyon.2020.e05701
Kurek K, Plitta-Michalak B, Ratajczak E (2019) Reactive oxygen species as potential drivers of the seed aging process. Plants 8(6):174. https://doi.org/10.3390/plants8060174
Law MY, Charles SA, Halliwell B (1983) Glutathione and ascorbic acid in spinach (Spinacia oleracea) Chloroplasts. J Biochem 210(3):899–903
Li W, Niu Y, Zheng Y, Wang Z (2022) Advances in the understanding of reactive oxygen species-dependent regulation on seed dormancy, germination, and deterioration in crops. Front Plant Sci 13:826809
Liu Y, Ye N, Liu R, Chen M, Zhang J (2010) H2O2 mediates the regulation of ABA catabolism and GA biosynthesis in Arabidopsis seed dormancy and germination. J Exp Bot 61(11):2979–2990
Ma Y, Zhu M, Shabala L et al (2016) Conditioning of roots with hypoxia increases aluminum and acid stress tolerance by mitigating activation of K+ efflux channels by ROS in barley: insights into cross-tolerance mechanism. Plant Cell Physiol 57(1):160–173
MacNevin WM, Uron PF (1953) Spectrum of hydrogen peroxide from organic hydroperoxides. J Anal Chem 25(11):1760–1761
Mensor LI, Menezes FS, Leitao GG, Reis AS, dos Santos T, Coube CS et al (2001) Screening of Brazilian plant extracts for antioxidant activity by the use of DPPH free radical method. Phytother Res 15(2):127–130
Nakano Y, Asada K (1981) Hydrogen peroxide is scavenged by ascorbate specific peroxidase in spinach chloroplasts. Plant Cell Physiol 22:867–880
Pawar VA, Laware SL (2018) Seed priming a critical review. Int J Sci Res 5(5):94–101
Pfaffl MW (2001) A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acid Res 29(9):e45. https://doi.org/10.1093/nar/29.9.e45
Rajjou L, Duval M, Gallardo K, Catusse J, Bally J, Job C et al (2012) Seed germination and vigor. Ann Rev Plant Biol 63:507–533. https://doi.org/10.1146/annurevarplant-042811-105550
Ratajczak E, Małecka A, Bagniewska-Zadworna A, Kalemba EM (2015) The production, localization and spreading of reactive oxygen species contributes to the low vitality of long-term stored common beech (Fagus sylvatica L.) seeds. J Plant Physiol 174:147–156
Rubio-Casal AE, Castillo JM, Lucue CJ, Fig Ureo ME (2003) Influence of salinity on germination and seed viability of two primary colonizers of Mediterranean salt plants. J Arid Environ 53(2):145–152
Saravanan P (2017) GC-MS analysis of ethanolic flower extract of Lantana camara Linn (Verbenaceae). Int J Curr Res Chem Pharm Sci 4(11):34–42. https://doi.org/10.22192/ijcrcps.2017.04.11.006
Simontacchi M, Caro A, Fraga CG, Puntarulo S (1993) Oxidative stress affects α-tocopherol content in soyabean embryonic axes upon imbibitions. Plant Physiol 103:949–953
Snell FD, Snell CT (1971) Colorimetric methods of analysis. Van Nostard Reinford Co, New York
Sridharan R, Raja S, Sakthivel P (2015) Effect of triazole compounds on induced changes in growth biomass and biochemical content of white radish (Raphanus sativus L.). J Plant Stress Physiol 1:43–48
Tietze F (1969) Enzymatic method for quantitative determination of nanogram amounts of total and oxidised glutathione: application to mammalian blood and other tissues. Anal Biochem 27(3):502–522
Ved A, Arsi T, Prakash O, Gupta A (2018) A review on phytochemistry and pharmacological activity of Lantana camara Linn. Int J Pharm Sci Res 9(1):37–43. https://doi.org/10.13040/IJPSR.0975-8232
Wahid A, Khaliq S (2015) Architectural and biochemical changes in embryonic tissues of maize under cadmium toxicity. Plant Biol 17(5):1005–1012. https://doi.org/10.1111/plb.12326
Wang W, Liu S, Song S, Møller (2015a) Proteomics of seed development, desiccation tolerance, germination and vigor. Plant Physiol Biochem 86:1–15
Wang Y, Li Y, Xue H, Pritchard HW, Wang X (2015b) Reactive oxygen species (ROS)-provoked mitochondria-dependent cell death during aging of elm (Ulmus pumila L.) seeds. Plant J 81(3):438–452
Wojtyla L, Lechowska K, Kubala S, Garnczarska M (2016) Different modes of hydrogen peroxide action during seed germination. Front Plant Sci 7:66. https://doi.org/10.3389/fpls.2016.00066
Xia F, Chen L, Sun Y, Mao P (2015) Relationships between ultrastructure of embryo cells and biochemical variations during aging of oat (Avena sativa L.) seeds with different moisture content. Acta Physiol Plant 37:89. https://doi.org/10.1007/s11738-015-1825-8
Xu L, Xin X, Yin G, Zhou J, Zhou Y, Lu X (2020) Timing for antioxidant-priming against rice seed aging: optimal only in non-resistant stage. Sci Rep 10(1):13294
Yan H, Jia S, Mao P (2020) Melatonin priming alleviates aging-induced germination inhibition by regulating β-oxidation, protein translation, and antioxidant metabolism in oat (Avena sativa L.) seeds. Int J Mol Sci 21(5):1898. https://doi.org/10.3390/ijms21051898
Yang PM, Huang QC, Qin GY, Zhao SP, Zhou JG (2014) Different drought-stress responses in photosynthesis and reactive oxygen metabolism between auto tetraploid and diploid rice. Photosynthetica 52(2):193–202
Yasmeen A, Basra SMA, Ahmad R, Wahid A (2012) Performance of late sown wheat in response to foliar application of moringa oleifera lam. leaf extract. Chil J Agric Res 72:92–97
Zhang J, Kirkham MB (1996) Antioxidant responses to drought in sunflower and sorghum seedlings. New Phytol 132(3):361–373
Zhang K, Zhang Y, Sun J, Meng J, Tao J (2020) Deterioration of orthodox seeds during aging: Influencing factors, physiological alterations and the role of reactive oxygen species. Plant Physiol Biochem 158:475–485
Acknowledgements
BP acknowledges Council of Scientific and Industrial Research, New Delhi, India (File no. 09/025(0260)/2018-EMR-I) for her research fellowship. The financial help of Department of Science and Technology - Fund for Improvement of S&T Infrastructure (DST – FIST) (SR/FST/LS-1/2018/188©, dated 01.10.2019) for instrumentation facilities, also acknowledged gratefully. SB acknowledges Department of Science and Technology - Science and Engineering Research Board (DST – SERB) (CRG/2021/000513) for financial assistance.
Author information
Authors and Affiliations
Contributions
Study conception and design was done by SB. Material preparation, data collection and analysis were performed by BP, SB. The first draft of the manuscript was written by SB and all authors commented on previous versions of the manuscript. All authors approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
There is no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Supplementary Fig. 1
RP-HPLC chromatogram showing changes in endogenous titer of plant hormones (Gibberellic acid, Abscisic acid and Jasmonic acid) of potentiated-accelerated aged seeds [Herbal (c, h), H2O2 (d, i) and Triadimefon (e, j)] vis-a-vis untreated control (a, f) and non-potentiated-accelerated aged seeds (b, g) during early germination of two experimental IARCs (Oryza sativa L., Cultivar Tulaipanji and Jamainadu). Supplementary Fig. 2 Seed potentiation impact [Hydro-primed (c, i), Herbal (d, j), H2O2 (e, k) and Triadimefon (f, l)] of accelerated aged seeds vis-a-vis untreated control (a, g) and non-potentiated-accelerated aged seeds (b, h) on germination phenotypes of two experimental IARCs (Oryza sativa L., Cultivars Tulaipanji and Jamainadu).
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Pal, B., Bhattacharjee, S. Herbal and chemical seed potentiations improve the redox health of aged seeds of indigenous aromatic rice cultivars through regulation of oxidative window, gene expression, and restoration of hormonal homeostasis. Physiol Mol Biol Plants 29, 1269–1288 (2023). https://doi.org/10.1007/s12298-023-01375-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12298-023-01375-9