Abstract
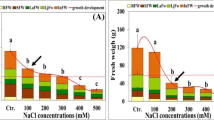
The natural capacity of plants to endure salt stress is largely regulated by multifaceted structural and physio-biochemical modulations. Salt toxicity endurance mechanism of six ecotypes of Typha domingensis Pers. was evaluated by analyzing photosynthesis, ionic homeostasis, and stomatal physiology under different levels of salinity (0, 100, 200 and 300 mM NaCl). Typha populations were collected across different areas of Punjab, an eastern province in Pakistan. All studied attributes among ecotypes presented differential changes as compared to control. Different salt treatments not only affected gas exchange attributes but also shown significant modifications in stomatal anatomical changes. As compared to control, net photosynthetic rate, transpiration rate, total chlorophyll contents and carotenoids were increased by 111%, 64%, 103% and 171% respectively, in Sahianwala ecotype among all other ecotypes. Similarly, maximum water use efficiency (WUE), sub stomatal CO2 concentration, sodium (Na+) and chloride (Cl−) contents were observed in Sahianwala (191%, 93%, 168%, 158%) and Knotti (162%, 75%, 146%, 182%) respectively, as compared to the others ecotypes. Adaxial and abaxial stomatal areas remained stable in Sahianwala and Knotti. The highest abaxial stomatal density was observed in Gatwala ecotype (42 mm2) and maximum adaxial stomatal density was recorded in Sahianwala ecotype (43 mm2) at 300 mM NaCl salinity. The current study showed that Typha ecotypes responded varyingly to salinity in terms of photosynthesis attributes to avoid damages due to salinity. Overall, differential photosynthetic activity, WUE, and changes in stomatal attributes of Sahianwala and Knotti ecotypes contributed more prominently in tolerating salinity stress. Therefore, Typha domingensis is a potential species to be used to rehabilitate salt affected lands for agriculture and aquatic habitat.









Similar content being viewed by others
References
Acosta-Motos JR, Ortuño MF, Bernal-Vicente A, Diaz-Vivancos P, Sanchez-Blanco MJ, Hernandez JA (2017) Plant responses to salt stress: adaptive mechanisms. Agronomy 7(1):18
Agarwal PK, Shukla PS, Gupta K, Jha B (2013) Bioengineering for salinity tolerance in plants: state of the art. Mol Biotechnol 54(1):102–123
Ali AM (2018) Nutrient sufficiency ranges in mango using boundary-line approach and compositional nutrient diagnosis norms in El-Salhiya, Egypt. Commun Soil Sci Plant Anal 49(2):188–201. https://doi.org/10.1080/00103624.2017.1421651
Aqeel M, Khalid N, Tufail A, Ahmad RZ, Akhter MS, Luqman M, Javed MT, Irshad MK, Alamri S, Hashem M, Noman A (2021) Elucidating the distinct interactive impact of cadmium and nickel on growth, photosynthesis, metal-homeostasis, and yield responses of mung bean (Vigna radiata L.) varieties. Environ Sci Pollut Res. https://doi.org/10.1007/s11356-021-12579-5
Arnon DI (1949) Copper enzymes in isolated chloroplasts. Polyphenoloxidase in Beta vulgaris. Plant Physiol 24(1):1
Ashraf M (2004) Some important physiological selection criteria for salt tolerance in plants. Flora Morphol Distrib Funct Eco Plants 199(5):361–376
Cuin TA, Tian Y, Betts SA, Chalmandrier R, Shabala S (2009) Ionic relations and osmotic adjustment in durum and bread wheat under saline conditions. Funct Plant Biol 36(12):1110–1119
da Cunha Cruz Y, Scarpa ALM, Pereira MP, de Castro EM, Pereira FJ (2020) Root anatomy and nutrient uptake of the cattail Typha domingensis Pers. (Typhaceae) grown under drought condition. Rhizosphere 16:100253
Davies B (1976) Chemistry and biochemistry of plant pigments. Carotenoids 2:38–165
de Andrade Silva FK, dos Santos Magalhães C, Sá RD, Carolina F, da Silva L, Randau KP (2020) Anatomical and histochemical study of Sechium edule (Jacq.) Sw. In: Anales de Biología. Servicio de Publicaciones de la Universidad de Murcia, pp 173–181
Ditta A, Imtiaz M, Mehmood S, Rizwan MS, Mubeen F, Aziz O, Qian Z, Ijaz R, Tu S (2018) Rock phosphate-enriched organic fertilizer with phosphate-solubilizing microorganisms improves nodulation, growth, and yield of legumes. Commun Soil Sci Plant Anal 49(21):2715–2725
Franks PJ, Farquhar GD (2007) The mechanical diversity of stomata and its significance in gas-exchange control. Plant Physiol 143(1):78–87
Hameed M, Ashraf M (2008) Physiological and biochemical adaptations of Cynodon dactylon (L.) Pers. from the salt range (Pakistan) to salinity stress. Flora Morphol Distrib Funct Eco Plants 203(8):683–694
Hameed M, Ashraf M, Naz N, Al-Qurainy F (2010) Anatomical adaptations of Cynodon dactylon (L.) Pers. from the salt range Pakistan to salinity stress. I. Root and stem anatomy. Pak J Bot 42(1):279–289
Hoagland DR, Arnon DI (1950) The water-culture method for growing plants without soil. In: Circular. California agricultural experiment station, vol 347, 2nd edn. College of Agriculture, University of California, Berkeley
Horie T, Karahara I, Katsuhara M (2012) Salinity tolerance mechanisms in glycophytes: an overview with the central focus on rice plants. Rice 5(1):1–18
Horneck D, Hanson D (1998) Determination of potassium and sodium by flame emission spectrophotometry. In: Karla YP (ed) Handbook of reference methods for plant analysis, vol 19. CRC Press, Washington, pp 153–155
Hussain T, Tan B, Yin Y, Blachier F, Tossou MC, Rahu N (2016) Oxidative stress and inflammation: what polyphenols can do for us? Oxid Med Cell Longev 2016:7432797
Jackson ML (1962) Soil chemical analysis-advanced course, vol 497. Dept. of Soil Science, Univ. of Wisconsin Madison, Wisconsin
Jiang J-L, Tian Y, Li L, Yu M, Hou R-P, Ren X-M (2019) H2S alleviates salinity stress in cucumber by maintaining the Na+/K+ balance and regulating H2S metabolism and oxidative stress response. Front Plant Sci. https://doi.org/10.3389/fpls.2019.00678
Johansen DA (1940) Plant microtechnique. McGraw-Hill Book Company Inc, London, p 530p
Jordan F, Waugh WJ, Glenn EP, Sam L, Thompson T, Thompson TL (2008) Natural bioremediation of a nitrate-contaminated soil-and-aquifer system in a desert environment. J Arid Environ 72(5):748–763
Khalid N, Rizvi ZF, Yousaf N, Khan SM, Noman A, Aqeel M, Latif K, Rafique A (2021) Rising metals concentration in the environment: a response to effluents of leather industries in Sialkot. Bull Environ Contam Toxicol. https://doi.org/10.1007/s00128-021-03111-z
Khan MA, Gul B (2002) Some ecophysiological aspects of seed germination in halophytes. Halophyte Util Reg Sustain Dev Agric 56–68
Kim H-Y (2014) Analysis of variance (ANOVA) comparing means of more than two groups. Restor Dent Endod 39(1):74
Kraus JE, Arduin M (1997) Manual básico de métodos em morfologia vegetal (basic manual of methods in plant morphology), vol 1. Edur Rio de Janeiro, Seropedica
Munns R (2002) Salinity, growth and phytohormones. In: Salinity: environment-plants-molecules. Springer, pp 271–290
Munns R, Tester M (2008) Mechanisms of salinity tolerance. Annu Rev Plant Biol 59:651–681
Naseer M, Hameed M, Zahoor A, Ahmad F, Fatima S, Ahmad MSA, Ahmad KS, Iftikhar M (2017) Photosynthetic response in buttonwood (Conocarpus erectus L.) to salt stress. Pak J Bot 49(3):847–856
Naz N, Hameed M, Ashraf M, Al-Qurainy F, Arshad M (2010) Relationships between gas-exchange characteristics and stomatal structural modifications in some desert grasses under high salinity. Photosynthetica 48(3):446–456
Negrão S, Schmöckel S, Tester M (2017) Evaluating physiological responses of plants to salinity stress. Ann Bot 119(1):1–11
Nilson SE, Assmann SM (2007) The control of transpiration. Insights from Arabidopsis. Plant Physiol 143(1):19–27
Noman A, Hameed M, Ali Q, Aqeel M (2012) Foliar tissue architectural diversity among three species of genus Hibiscus for better adaptability under industrial environment. Int J Envrion Sci 2(4):2212–2222
Noman A, Aqeel M, Javed M, Zafar S, Ali Q, Islam W, Irshad M, Buriro M, Kanwal H, Khalid N (2017) Histological changes in Hibiscus rosa-sinensis endorse acclimation and phytoremediation of industrially polluted sites. J Anim Plant Sci 27(5):1637–1648
Noreen S, Akhter MS, Yaamin T, Arfan M (2018) The ameliorative effects of exogenously applied proline on physiological and biochemical parameters of wheat (Triticum aestivum L.) crop under copper stress condition. J Plant Interact 13(1):221–230
Noreen S, Faiz S, Akhter MS, Shah KH (2019) Influence of foliar application of osmoprotectants to ameliorate salt stress in sunflower (Helianthus annuus L.). Sarhad J Agric 35(4):1316–1325
Oliveira J, Pereira M, Duarte V, Corrêa F, Castro E, Pereira F (2018) Cadmium tolerance of Typha domingensis Pers. (Typhaceae) as related to growth and leaf morphophysiology. Braz J Biol 78(3):509–516
OriginLab Corporation (2014) OriginPro 9.1: scientific data analysis and graphing software. Northampton, MA 01060, United States. https://www.originlab.com. Assessed 15 Nov 2020
Parkhurst DF (1994) Diffusion of CO2 and other gases inside leaves. New Phytol 126(3):449–479
Phang TH, Shao G, Lam HM (2008) Salt tolerance in soybean. J Integr Plant Biol 50(10):1196–1212
R Development Core Team (2017) R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. http://www.R-project.org. Accessed 15 Nov 2020
Robinson MF, Heath J, Mansfield T (1998) Disturbances in stomatal behaviour caused by air pollutants. J Exp Bot 49:461–469
Shabala S, Hariadi Y, Jacobsen S-E (2013) Genotypic difference in salinity tolerance in quinoa is determined by differential control of xylem Na+ loading and stomatal density. J Plant Physiol 170(10):906–914
Steel RG, Torrie JH, Dickey DA (1997) Principles and procedures of statistics. McGraw-Hill, New York
Tavakkoli E, Rengasamy P, McDonald GK (2010) High concentrations of Na+ and Cl− ions in soil solution have simultaneous detrimental effects on growth of faba bean under salinity stress. J Exp Bot 61(15):4449–4459. https://doi.org/10.1093/jxb/erq251
Tucker B, Kurtz L (1961) Calcium and magnesium determinations by EDTA titrations. Soil Sci Soc Am J 25(1):27–29
Tufail A, Aqeel M, Khalid N, Ahsan M, Khalil S, Ahmad F, Hameed M, Noman A, Alamri S, Hashem M (2020) Salt toxicity in a natural habitat induces structural and functional modifications and modulate metabolism in bermuda grass (Cynodon dactylon [L.] Pers.). Appl Ecol Environ Res 18(5):6569–6588
Wolf B (1982) A comprehensive system of leaf analyses and its use for diagnosing crop nutrient status. Commun Soil Sci Plant Anal 13(12):1035–1059
Wu L (1981) The potential for evolution of salinity tolerance in Agrostis stolonifera L. and Agrostis tenuis Sibth. New Phytol 89(3):471–486
Yang H, Zhang X, Wang G (2004) Relationships between stomatal character, photosynthetic character and seed chemical composition in grass pea at different water availabilities. J Agric Sci 142:675
Yang G, Li F, Tian L, He X, Gao Y, Wang Z, Ren F (2020) Soil physicochemical properties and cotton (Gossypium hirsutum L.) yield under brackish water mulched drip irrigation. Soil Till Res 199:104592
Yoo CY, Pence HE, Hasegawa PM, Mickelbart MV (2009) Regulation of transpiration to improve crop water use. Crit Rev Plant Sci 28(6):410–431
Zhu M, Shabala S, Shabala L, Fan Y, Zhou M (2016) Evaluating predictive values of various physiological indices for salinity stress tolerance in wheat. J Agron Crop Sci 202(2):115–124
Zulfiqar F, Akram NA, Ashraf M (2020a) Osmoprotection in plants under abiotic stresses: new insights into a classical phenomenon. Planta 251(1):3
Zulfiqar F, Younis A, Riaz A, Mansoor F, Hameed M, Akram NA, Abideen Z (2020b) Morpho-anatomical adaptations of two Tagetes erecta L. cultivars with contrasting response to drought stress. Pak J Bot 52(3):801–810
Acknowledgements
The authors extend their appreciation to the deanship of scientific research, King Khalid University for funding work through research group program under Grant R.G.P. 2/11/42
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Akhter, N., Aqeel, M., Shahnaz, M.M. et al. Physiological homeostasis for ecological success of Typha (Typha domingensis Pers.) populations in saline soils. Physiol Mol Biol Plants 27, 687–701 (2021). https://doi.org/10.1007/s12298-021-00963-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12298-021-00963-x