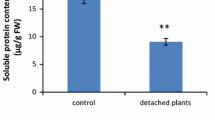
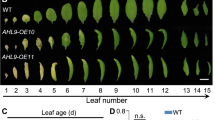
Abstract
Leaf senescence is an important developmental process for the plant life cycle. It is controlled by endogenous and environmental factors and can be positively or negatively affected by plant growth regulators. It is characterised by major and significant changes in the patterns of gene expression. Auxin, especially indole-3-acetic acid (IAA), is a plant growth hormone that affects plant growth and development. The effect of IAA on leaf senescence is still unclear. In this study, we performed microarray analysis to investigate the role of IAA on gene expression during senescence in Arabidopsis thaliana. We sprayed IAA on plants at 3 different time points (27, 31 or 35 days after sowing). Following spraying, PSII activity of the eighth leaf was evaluated daily by measurement of chlorophyll fluorescence parameters. Our results show that PSII activity decreased following IAA application and the IAA treatment triggered different gene expression responses in leaves of different ages.









Similar content being viewed by others
References
Allemeersch J, Durinck S, Vanderhaeghen R, Alard P, Maes R, Seeuws K et al (2005) Benchmarking the catma microarray: a novel tool for Arabidopsis transcriptome analysis. Plant Physiol 137:588–601
Baker NR, Rosenqvist E (2004) Applications of chlorophyll fluorescence can improve crop production strategies: an examination of future possibilities. J Exp Bot 55:1607–1621
Balazadeh S (2014) Stay-green not always stays green. Mol Plant 7:1264–1266
Barbagallo RP, Oxborough K, Pallett KE, Baker NR (2003) Rapid, noninvasive screening for perturbations of metabolism and plant growth using chlorophyll fluorescence imaging. Plant Physiol 132:485–493
Bashri G, Prasad SM (2016) Exogenous IAA differentially affects growth, oxidative stress and antioxidants system in Cd stressed Trigonella foenum-graecum L. seedlings: toxicity alleviation by up-regulation of ascorbate-glutathione cycle. Ecotoxicol Environ Saf 132:329–338
Breeze E, Harrison E, Page T, Warner N, Shen C, Zhang C, Buchanan-Wollaston V (2008) Transcriptional regulation of plant senescence: from functional genomics to systems biology. Plant Biol 10:99–109
Breeze E, Harrison E, McHattie S, Hughes L, Hickman R, Hill C et al (2011) High-resolution temporal profiling of transcripts during Arabidopsis leaf senescence reveals a distinct chronology of processes and regulation. Plant Cell 23:873–894
Buchanan-Wollaston V, Earl S, Harrison E, Mathas E, Navabpour S, Page T, Pink D (2003) The molecular analysis of leaf senescence—a genomics approach. Plant Biotechnol J 1:3–22
Buchanan-Wollaston V, Page T, Harrison E, Breeze E, Lim PO, Nam HG et al (2005) Comparative transcriptome analysis reveals significant differences in gene expression and signalling pathways between developmental and dark/starvation-induced senescence in Arabidopsis. The Plant J 42:567–585
Casazza AP, Rossini S, Rosso MG, Soave C (2005) Mutational and expression analysis of ELIP1 and ELIP2 in Arabidopsis thaliana. Plant Mol Biol 58:41–51
Chamarro J, Östin A, Sandberg G (2001) Metabolism of indole-3-acetic acid by orange (Citrus sinensis) flavedo tissue during fruit development. Phytochemistry 57(179):187
Chen W, Provart NJ, Glazebrook J, Katagiri F, Chang HS, Eulgem T et al (2002) Expression profile matrix of Arabidopsis transcription factor genes suggests their putative funcions in response to environmental stresses. Plant Cell 14:559–574
De Michele R, Vurro E, Rigo C, Costa A, Elviri L, Di Valentin M et al (2009) Nitric oxide is involved in cadmium-induced programmed cell death in Arabidopsis suspension cultures. Plant Physiol 150:217–228
Ellis CM, Nagpal P, Young JC, Hagen G, Guilfoyle TJ, Reed JW (2005) AUXIN RESPONSE FACTOR1 and AUXIN RESPONSE FACTOR2 regulate senescence and floral organ abscission in Arabidopsis thaliana. Development 132:4563–4574
Fan L, Zheng S, Wang X (1997) Antisense suppression of phospholipase D alpha retards abscisic acid-and ethylene-promoted senescence of postharvest Arabidopsis leaves. Plant Cell 9:2183–2196
Farage-Barhom S, Burd S, Sonego L, Perl-Treves R, Lers A (2008) Expression analysis of the BFN1 nuclease gene promoter during senescence, abscission, and programmed cell death-related processes. J Exp Bot 59:3247–3258
Fischer AM (2007) Nutrient remobilization during leaf senescence. In: Gan S (ed) Annual plant reviews volume 26: senescence processes in plants. https://doi.org/10.1002/9780470988855.ch5
Ghanem ME, Albacete A, Martínez-Andújar C, Acosta M, Romero-Aranda R, Dodd IC et al (2008) Hormonal changes during salinity-induced leaf senescence in tomato (Solanum lycopersicum L.). J Exp Bot 59:3039–3050
Goda H, Sawa S, Asami T, Fujioka S, Shimada Y, Yoshida S (2004) Comprehensive comparison of auxin-regulated and brassinosteroid-regulated genes in Arabidopsis. Plant Physiol 134:1555–1573
Gören N, Çağ S (2007) The effect of Indole-3-acetic acid and benzyladenine on sequential leaf senescence on Helianthus annuus L. seedlings. Biotechnol Biotechnol Equip 21:322–327
Gregersen PL, Culetic A, Boschian L, Krupinska K (2013) Plant senescence and crop productivity. Plant Mol Biol 82:603–622
Haldimann P, Fracheboud Y, Stamp P (1996) Photosynthetic performance and resistance to photoinhibition of Zea mays L. leaves grown at sub-optimal temperature. Plant Cell Environ 19:85–92
He Y, Fukushige H, Hildebrand DF, Gan S (2002) Evidence supporting a role of jasmonic acid in Arabidopsis leaf senescence. Plant Physiol 128:876–884
Hickman R, Hill C, Penfold CA, Breeze E, Bowden L, Moore JD et al (2013) A local regulatory network around three NAC transcription factors in stress responses and senescence in Arabidopsis leaves. The Plant J 75:26–39
Hu Y, Jiang Y, Han X, Wang H, Pan J, Yu D (2017) Jasmonate regulates leaf senescence and tolerance to cold stress: crosstalk with other phytohormones. J Exp Bot 68:1361–1369
Ji H, Wang S, Cheng C, Li R, Wang Z, Jenkins GI et al (2019) The RCC 1 family protein SAB 1 negatively regulates ABI 5 through multidimensional mechanisms during postgermination in Arabidopsis. New Phytol 222:907–922
Jiang Y, Liang G, Yang S, Yu D (2014) Arabidopsis WRKY57 functions as a node of convergence for jasmonic acid-and auxin-mediated signaling in jasmonic acid-induced leaf senescence. Plant Cell 26:230–245
Jibran R, Hunter DA, Dijkwel PP (2013) Hormonal regulation of leaf senescence through integration of developmental and stress signals. Plant Mol Biol 82:547–561
Jones DC, Zheng W, Huang S, Du C, Zhao X, Yennamalli RM et al (2016) A clade-specific Arabidopsis gene connects primary metabolism and senescence. Front Plant Sci 7:983–987
Ju L, Jing Y, Shi P, Liu J, Chen J, Yan J et al (2019) JAZ proteins modulate seed germination through interaction with ABI 5 in bread wheat and Arabidopsis. New Phytol 223:246–260
Jung H, Lee DK, Do Choi Y, Kim JK (2015) OsIAA6, a member of the rice Aux/IAA gene family, is involved in drought tolerance and tiller out growth. Plant Sci 236:304–312
Jusoh M, Loh SH, Chuah TS, Aziz A, San Cha T (2015) Indole-3-acetic acid (IAA) induced changes in oil content, fatty acid profiles and expression of four fatty acid biosynthetic genes in Chlorella vulgaris at early stationary growth phase. Phytochemistry 111:65–71
Karatas I, Ozturk L, Ersahin Y, Okatan Y (2010) Effects of auxin on photosynthetic pigments and some enzyme activities during dark-induced senescence of Tropaeolum leaves. Pak J Bot 42(3):1881–1888
Katari MS, Nowicki SD, Aceituno FF, Nero D, Kelfer J, Thompson LP et al (2010) VirtualPlant: a software platform to support systems biology research. Plant Physiol 152:500–515
Kim J, Kim JH, Lyu J, Woo HR, Lim PO (2018) New insights into regulation of leaf senescence in Arabidopsis. J Exp Bot 69:787–799
Li RH, Guo PG, Michael B, Stefania G, Salvatore C (2006) Evaluation of chlorophyll content and fluorescence parameters as indicators of drought tolerance in barley. Agr Sci China 5:751–757
Li W, Li X, Chao J, Zhang Z, Wang W, Guo Y (2018) NAC family transcription factors in tobacco and their potential role in regulating leaf senescence. Front Plant Sci 9:1900–1915
Lim PO, Nam HG (2007) Aging and senescence of the leaf organ. J Plant Biol 50:291–300
Lim PO, Kim HJ, Nam HG (2007) Leaf senescence. Annu Rev Plant Biol 58:115–136
Lopez-Molina L, Mongrand S, Kinoshita N, Chua NH (2003) AFP is a novel negative regulator of ABA signaling that promotes ABI5 protein degradation. Genes Dev 17:410–418
Lu C, Lu Q, Zhang J, Kuang T (2001) Characterization of photosynthetic pigment composition, photosystem II photochemistry and thermal energy dissipation during leaf senescence of wheat plants grown in the field. J Exp Bot 52:1805–1810
Morris K, Mackerness SAH, Page T, John CF, Murphy AM, Carr JP, Buchanan-Wollaston V (2000) Salicylic acid has a role in regulating gene expression during leaf senescence. The Plant J 23:677–685
Mueller-Roeber B, Balazadeh S (2014) Auxin and its role in plant senescence. J Plant Growth Regul 33:21–33
Perea-Resa C, Rodríguez-Milla MA, Iniesto E, Rubio V, Salinas J (2017) Prefoldins negatively regulate cold acclimation in Arabidopsis thaliana by promoting nuclear proteasome-mediated HY5 degradation. Mol Plant 10:791–804
Petti C, Nair M, DeBolt S (2014) The involvement of J-protein AtDjC17 in root development in Arabidopsis. Front Plant Sci 5:532–541
Rai MI, Wang X, Thibault DM, Kim HJ, Bombyk MM et al (2015) The ARGOS gene family functions in a negative feedback loop to desensitize plants to ethylene. BMC Plant Biol 15:157. https://doi.org/10.1186/s12870-015-0554-x
Reeves WM, Lynch TJ, Mobin R, Finkelstein RR (2011) Direct targets of the transcription factors ABA-Insensitive (ABI) 4 and ABI5 reveal synergistic action by ABI4 and several bZIP ABA response factors. Plant Mol Biol 75:347–363
Rodríguez-Milla MA, Salinas J (2009) Prefoldins 3 and 5 play an essential role in Arabidopsis tolerance to salt stress. Mol Plant 2:526–534
Rouse D, Mackay P, Stirnberg P, Estelle M, Leyser O (1998) Changes in auxin response from mutations in an AUX/IAA gene. Science 279:1371–1373
Sağlam-Çağ S, Okatan Y (2014) The effects of zinc (Zn) and C14-indoleacetic acid (IAA) on leaf senescence ın Helianthus annuus L. Int J Plant Physiol Biochem 6:28–33
Sanjari S, Shirzadian-Khorramabad R, Shobbar Z-S, Shahbazi M (2019) Systematic analysis of NAC transcription factors’ gene family and identification of post-flowering drought stress responsive members in sorghum. Plant Cell Rep 38:361–376
Sarwat M, Naqvi AR, Ahmad P, Ashraf M, Akram NA (2013) Phytohormones and microRNAs as sensors and regulators of leaf senescence: assigning macro roles to small molecules. Biotechnol Adv 31:1153–1171
Sghaier N, Ayed RB, Gorai M, Rebai A (2018) Prediction of auxin response elements based on data fusion in Arabidopsis thaliana. Mol Biol Rep 45:763–772
Sharabi-Schwager M, Samach A, Porat R (2010) Overexpression of the CBF2 transcriptional activator in Arabidopsis suppresses the responsiveness of leaf tissue to the stress hormone ethylene. Plant Biol 12:630–638
Shi H, Reiter RJ, Tan DX, Chan Z (2015) INDOLE- 3- ACETIC ACID INDUCIBLE 17 positively modulates natural leaf senescence through melatonin- mediated pathway in Arabidopsis. J Pineal Res 58:26–33
Song C, Chung WS, Lim CO (2016) Overexpression of heat shock factor gene HsfA3 increases galactinol levels and oxidative stress tolerance in Arabidopsis. Mol Cells 39:477–479
Srivastava LM (2002) Plant growth and development Hormones and Environment. Academic Press, California. ISBN 0-12-660570-X
Thakur JK, Jain M, Tyagi AK, Khurana JP (2005) Exogenous auxin enhances the degradation of a light down-regulated and nuclear-localized OsiIAA1, an Aux/IAA protein from rice, via proteasome. Biochim Biophys Acta Gene Struct Expr 1730:196–205
Ueda H, Kusaba M (2015) Strigolactone regulates leaf senescence in concert with ethylene in Arabidopsis. Plant Physiol 169:138–147
van der Graaff E, Schwacke R, Schneider A, Desimone M, Flügge UI, Kunze R (2006) Transcription analysis of Arabidopsis membrane transporters and hormone pathways during developmental and induced leaf senescence. Plant Physiol 141:776–792
Wilson R, Kim H, Bakshi A, Binder B (2014) The ethylene receptors, ETHYLENE RESPSONE 1 and 2, have contrasting roles in seed germination of Arabidopsis thaliana during salt stress. Plant Physiol 165:1353–1366
Wu A, Allu AD, Garapati P, Siddiqui H et al (2012) JUGBURUNNEN1, a reactive oxygen species-responsive NAC transcription factor, regulates longevity in Arabidopsis. Plant Cell 24:482–506
www.arabidopsis.org. Accessed 10 April 2019
Wyatt RE, Ainley WM, Nagao RT, Conner TW, Key JL (1993) Expression of the Arabidopsis AtAux2-11 auxin-responsive gene in transgenic plants. Plant Mol Biol 22:731–749
Yamada Y, Umehara M (2015) Possible roles of strigolactones during leaf senescence. Plants 4:664–677
Yamada Y, Furusawa S, Nagasaka S, Shimomura K, Yamaguchi S, Umehara M (2014) Strigolactone signaling regulates rice leaf senescence in response to a phosphate deficiency. Planta 240:399–408
Yao Y, You J, Ou Y, Ma J, Wu X, Xu G (2015) Ultraviolet-B protection of ascorbate and tocopherol in plants related with their function on the stability on carotenoid and phenylpropanoid compounds. Plant Physiol Biochem 90:23–31
Zhao Y (2010) Auxin biosynthesis and its role in plant development. Annu Rev Plant Biol 61:49–64
Zhao Y, Tian X, Li Y, Zhang L, Guan P, Kou X et al (2017) Molecular and functional characterization of wheat ARGOS genes influencing plant growth and stress tolerance. Front Plant Sci 8:170–177
Acknowledgements
This work is part of N.G-S’ PhD. thesis.
Author information
Authors and Affiliations
Contributions
N.G.-S., V.B.-W., E.H., E.B. and G.Ö designed the research; N.G.-S. did the experimental work, performed data analysis, wrote the paper, revised manuscript discussed with V.B.-W.
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
12298_2019_752_MOESM2_ESM.xlsx
Supplementary Table: Gene Ontology analysis of genes differentially expressed following IAA treatment. The previously reported relationship between the genes and senescence related expression was investigated using Mueller-Roeber and Balazadeh (2014) and column D and E indicate whether each gene was identified in this paper as being a senescence-associated gene (SAGs) or senescence downregulated gene (SDG), marked as (+) and (-), respectively. A: Genes within Ontology groups that were identified as being overrepresented amongst genes showing increased expression at following IAA treatment at 27, 31 and 35 DAS. B: Genes within Ontology groups that were identified as being overrepresented amongst genes showing decreased expression at following IAA treatment at 27, 31 and 35 DAS (XLSX 22 kb)
Rights and permissions
About this article
Cite this article
Gören-Sağlam, N., Harrison, E., Breeze, E. et al. Analysis of the impact of indole-3-acetic acid (IAA) on gene expression during leaf senescence in Arabidopsis thaliana. Physiol Mol Biol Plants 26, 733–745 (2020). https://doi.org/10.1007/s12298-019-00752-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12298-019-00752-7