Abstract
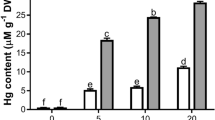
The phytotoxicity and oxidative damage in response to different concentrations of Hg (0.0, 2.5, 5.0, 10 and 25 μM) were evaluated in wheat plants. The root and shoot growth, content of chlorophyll and total soluble protein declined at 10 and 25 μM Hg. Roots of the plant were more affected as compared to the shoot. The malondialdehyde (MDA) quantity enhanced in the roots of wheat plants treated with 10 and 25 μM Hg and in the leaves of plants treated with 25 μM Hg. The concentration of H2O2 decreased at low concentration and increased at high concentration of Hg. The induction of enzymatic antioxidants (catalase, CAT; ascorbate peroxidase, APX; peroxidase, POX and superoxide dismutase, SOD) was found in the roots and leaves of plants with increased concentration of Hg up to 10 μM and low activities of these enzymes were observed at 25 μM Hg. Also, the level of K, Ca and Mg declined in leaf tissues of Hg treated plants. Thus wheat plants exposed to lower concentrations of Hg did not experience any oxidative stress. However, on treatment with 10 μM Hg, the roots and leaves responded differently. Both the leaves and roots of plants treated with higher concentration of Hg were subjected to comparatively greater oxidative damage and demonstrated that the antioxidative components were not able to remove the stress due to higher concentration of Hg and thus might affect the productivity in wheat plants.









Similar content being viewed by others
References
Aebi HE (1983) Catalase. In: Bergemeyer HU (ed) Methods of enzymatic analysis. Weinheim, Verlag Chemie, pp 273–285
Aina R, Labra M, Fumagalli P, Vannini C, Marsoni M (2007) Thiol-peptide level and proteomic changes in response to cadmium toxicity in Oryza sativa L. roots. Environ Exp Bot 59:381–392
Bradford M (1976) A rapid and sensitive method for quantification of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72:248–254
Carbonell-Barrachina AA, Arabi MA, Delaune RD, Gambrell RP, Patrick WH (1998) Arsenic in wetland vegetation: availability, phytotoxicity, uptake and effects on plant growth and nutrition. Sci Total Environ 217:189–199
Chaudhuri K, Chaudhuri MA (1993) Effects of short term NaCl salinity stress on free radicals mediated membrane damage in two jute species. Indian J Exp Biol 3:327–331
Cho U, Park J (1999) Changes in hydrogen peroxide content and activities of antioxidant enzymes in tomato seedlings exposed to mercury. J Plant Biol 42:41–48
Cho U, Park J (2000) Mercury-induced oxidative stress in tomato seedlings. Plant Sci 156:1–9
Foyer CH, Halliwell B (1976) The presence of glutathione and glutathione reductase in chloroplasts: a proposed role in ascorbic acid metabolism. Planta 133:21–25
Fridovich I (1986) Biological effects of the superoxide radical. Arch Biochem Biophys 24:1–11
Gong M, Li YJ, Chen SZ (1998) Abscisic acid induced thermotolerance in maize seedlings is mediated by calcium and associated with antioxidant system. J Plant Physiol 153:488–496
Gupta M, Chandra P (1998) Bioaccumulation and toxicity of mercury in rooted-submerged macrophyte Vallisneria spiralis. Environ Pollut 103:327–332
Heath RL, Packer L (1968) Photooxidation in isolated chloroplast. I. Kinetics and stoichiometry of fatty acid peroxidation. Arch Biochem Biophys 125:189–194
Hoagland DR, Arnon DI (1950) The water culture method of growing plant without soil. Calif Agr Expt Stat Circ 374:1–32
Israr M, Sahi S, Datta R, Sarkar D (2006) Bioaccumulation and physiological effects of mercury in Sesbania drummonii. Chemosphere 65:591–598
Jacques-Silva MC, Nogueira CW, Broch LC, Flores EMM, Rocha JBT (2001) Diphenyl diselenide and ascorbic acid changes deposition of selenium and ascorbic acid in liver and brain of mice. Pharmacol Toxicol 88:119–125
Kupper H, Kupper F, Spiller M (1998) In situ detection of heavy metal substituted chlorophylls in water plants. Photosyn Res 58:123–133
Liu D, Wang X, Chen Z, Xu H, Wang Y (2010) Influence of mercury on chlorophyll content in winter wheat and mercury bioaccumulation. Plant Soil Environ 56:139–143
Loreto F, Velikova V (2001) Isoprene produced by leaves protects the photosynthetic apparatus against ozone damage, quenches ozone products, and reduces lipid peroxidation of cellular membranes. Plant Physiol 127:1781–1787
Lozano-Rodriguez E, Luguera M, Lucena JJ, Carpena-Ruiz RO (1995) Evaluation of two different acid digestion methods in closed systems of trace element determination in plants. Quim Anal 14:27–30
Marklund S, Marklund G (1974) Involvement of the superoxide anion radical in the auto oxidation of pyrogallol and a convenient assay for superoxide dismutase. Eur J Biochem 47:469–474
McLaughlin MJ, Tiller KG, Naidu R, Stevens DP (1996) The behaviour and environmental impact of contaminants in fertilizers. Aust J Soil Res 34:1–54
Mishra A, Choudhuri MA (1999) Effects of salicylic acid on heavy metal-induced membrane deterioration mediated by lipoxygenase in rice. Biol Plant 42:409–415
Mittler R (2002) Oxidative stress, antioxidants and stress tolerance. Trends Plant Sci 7:405–410
Moreno-Jimenz E, Penalosa JM, Esteban E, Carpena Ruiz RO (2007) Mercury accumulation and resistance to mercury stress in Rumex induratus Marrubium vulgare grown in perlite. J Plant Nutr Soil Sci 170:485–494
Mukherjee AK, De B (1996) Hg induced metabolic changes in seedlings of cultivated cells of tomato. Geobios 23:83–88
Mukherji S, Mukherji C (1979) Characterization of cadmium effects in different plant materials. Indian J Exp Biol 17:265–269
Ortega-Villasante C, Rellan-Alvarez R, del Campo FF et al (2005) Cellular damage induced by cadmium and mercury in Medicago sativa. J Exp Bot 56:2239–2251
Patra M, Sharma A (2000) Mercury toxicity in plants. Bot Review 66:379–422
Porra RJ, Thompson WA, Kriedemann PE (1989) Determination of accurate extinction coefficients and simultaneous equations for assaying chlorophylls a and b extracted with four different solvents: verification of the concentration of chlorophyll standards by atomic absorption spectroscopy. Biochim Biophys Acta 975:384–394
Prasad MNV (2003) Biomarkers. In: Prasad MNV, Hagemeyer J (eds) Heavy metal stress in plants from molecules to ecosystem. Springer Verlag, Berlin, pp 445–448
Prasad DDK, Prasad ARK (1987) Altered δ-aminolevulenicacid metabolism by lead and mercury in germinating seedlings of bajra (Pennisetum typhoideum). J Plant Physiol 127:241–249
Rao MV, Paliyath G, Ormrod DP, Murr DP, Watkins CB (1997) Influence of salicylic acid on H2O2 production, oxidative stress and H2O2-metabolizing enzymes. Plant Physiol 115:137–149
Scandalios JG (1993) Regulation and properties of plant catalases. In: Foyer CH, Mullineaux PM (eds) Causes of photooxidative stress and amelioration of defense systems in plants. CRC Press, Boca Raton, pp 275–315
Schutzendubel A, Polle A (2002) Plant responses to abiotic stresses: heavy metal-induced oxidative stress and protection by mycorrhization. J Exp Bot 53:1351–1365
Shiyab S, Chen J, Fengxiang XH, David LM, Fank BM, Mengmeng G, Yi S, Motasim AM (2008) Mercury-induced oxidative stress in Indian mustard (Brassica juncea L.). Environ Toxicol 24:462–471
Shriapanahi M, Anderson AC (1986) Accumulation of Cd, Hg, Pb by vegetables following long-term land application of waste water. Sci Total Environ 52:41–48
Skrebsky EC, Tabaldi LA, Pereira LB, Rauber R, Maldaner J, Cargnelutti D, Gonc¸alves JF, Castro GY, Schetinger MRC, Nicoloso FT (2008) Effect of cadmium on growth, micronutrient concentration, and d-aminolevulinic acid dehydratase and acid phosphatase activities in plants of Pfaffia glomerata. Braz J Plant Physiol 20:285–294
Srivastava M, Ma LQ, Singh N, Singh S (2005) Antioxidant responses of hyperaccumulator and sensitive fern species to arsenic. J Exp Bot 56:1335–1342
Suszcynsky EM, Shann JR (1995) Phytotoxity and accumulation of mercury in tobacco subjected to different exposure routes. Environ Toxicol Chem 14:61–67
Ushimaru T, Kanematsu S, Shibasaka M, Tsuji H (1999) Effect of hypoxia on antioxidant enzymes in aerobically grown rice (Oryza sativa) seedlings. Physiol Plant 107:181–187
Varshney AK (1991) Phytotoxic effects of mercuric acetate on the development of chlorophyll in excised cotyledons of two cucurbits. Geobios 18:119–124
Vizarova G, Zatkalikova T, Zelenakoua E (1985) Effects of Hg on some physiological processes in barley. Biologia 50:573–576
Wellburn AR (1994) The spectral determination of chlorophyll a and b, as well as total carotenoids, using various solvents with spectrophotometers of different resolution. J Plant Physiol 144:307–313
Xylander M, Hagen C, Braune W (1996) Mercury increases light susceptibility in the green alga Haematococcus lacustris. Botanica Acta 109:222–228
Zhou ZS, Huang SQ, Guo K, Mehta SK, Zhang PC, Yang ZM (2007) Metabolic adaptations to mercury-induced oxidative stress in roots of Medicago sativa L. Inorg Biochem 101:1–9
Zhou ZS, Guo K, Elbaz AA, Yang ZM (2009) Salicylic acid alleviates mercury toxicity by preventing oxidative stress in roots of Medicago sativa. Environ Exp Bot 65:27–34
Zornoza P, Vazquez S, Esteban E, Fernandez-Pascual M, Carpena R (2002) Cadmium-stress in nodulated white lupin: strategies to avoid toxicity. Plant Physiol Biochem 40:1003–1009
Acknowledgements
We wish our sincere thanks to the authorities of MATS University for providing us laboratory facilities to carry out this work.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Sahu, G.K., Upadhyay, S. & Sahoo, B.B. Mercury induced phytotoxicity and oxidative stress in wheat (Triticum aestivum L.) plants. Physiol Mol Biol Plants 18, 21–31 (2012). https://doi.org/10.1007/s12298-011-0090-6
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12298-011-0090-6